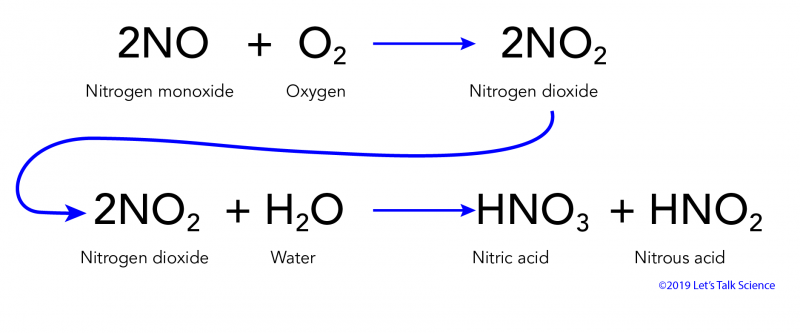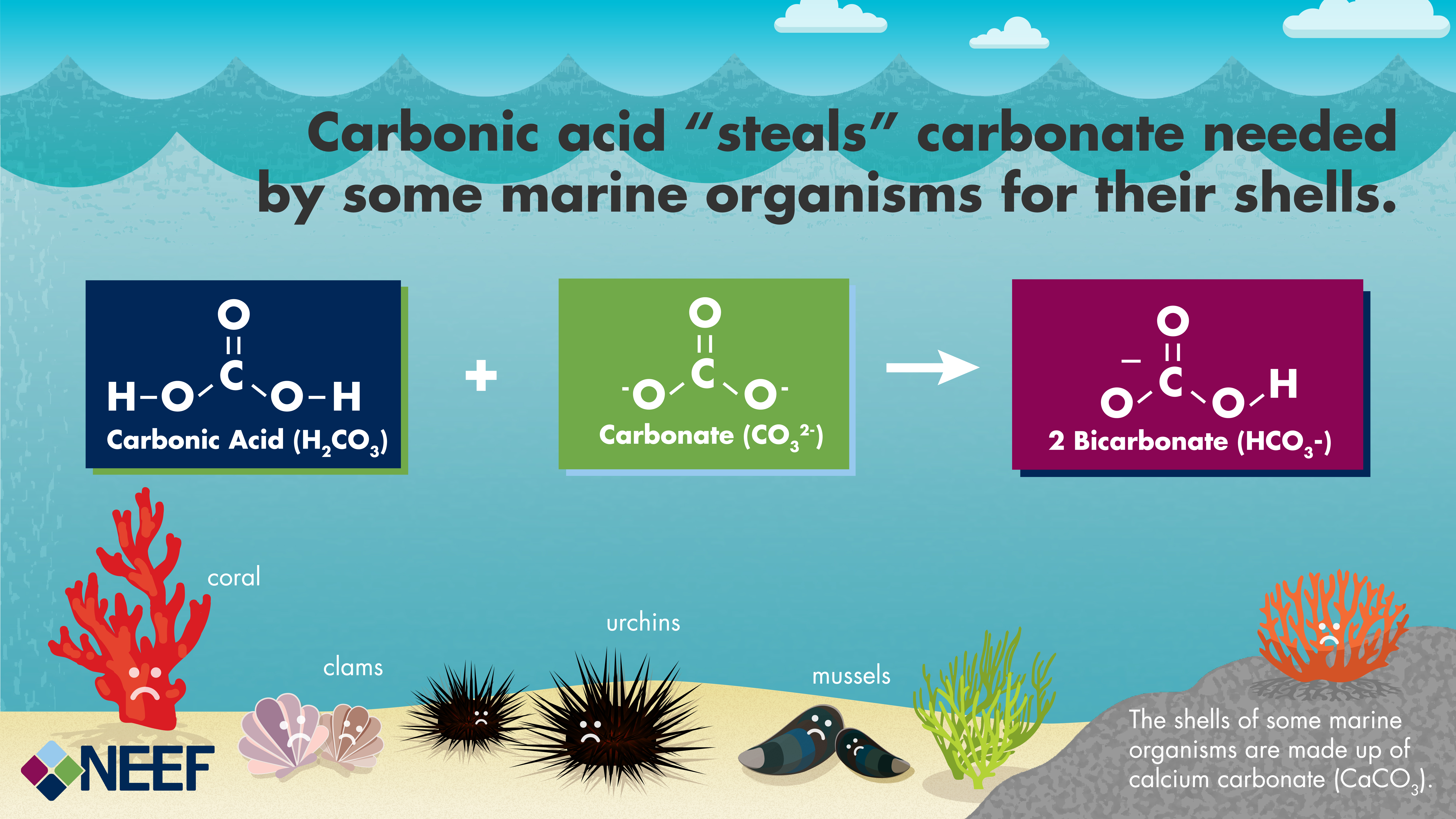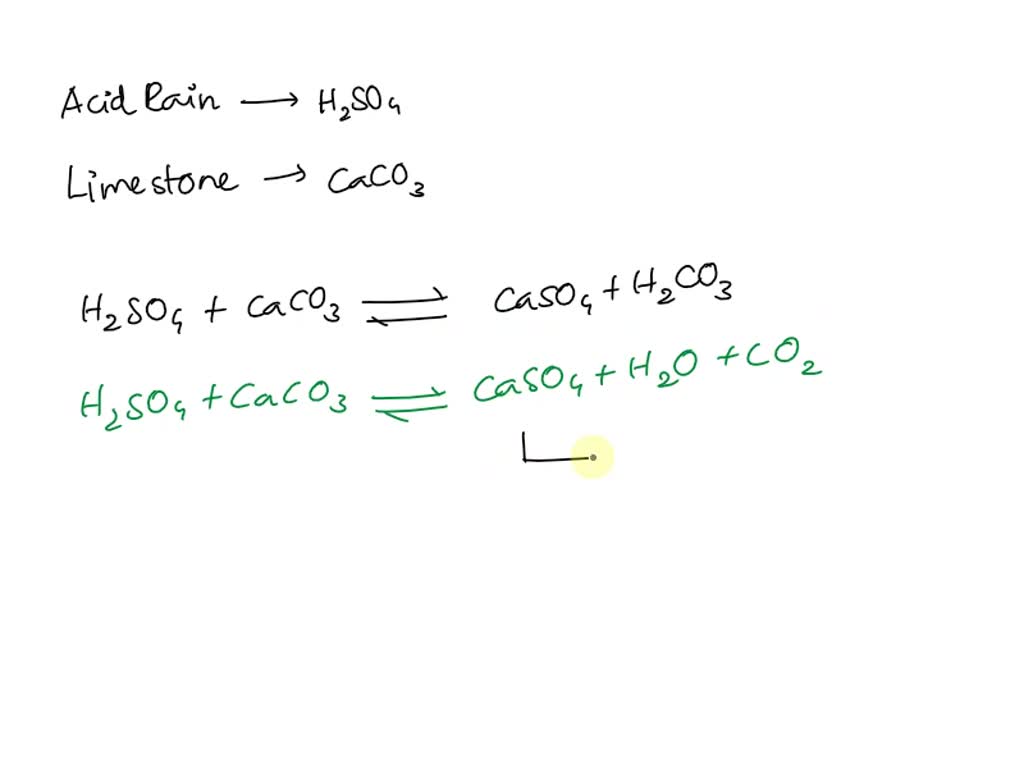
SOLVED: Acid rain is a dilute solution of acids that dissolve the calcium carbonate in limestone statues. Concentrated acids can dissolve a large piece of limestone in a few days. Statue breakdown
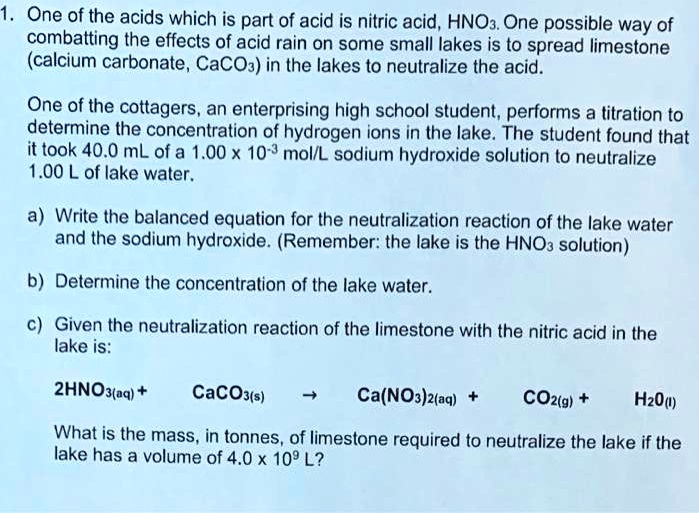
SOLVED: One of the acids which is part of acid is nitric acid, HNO: One possible way of combatting the effects of acid rain on some smali lakes is 0 spread limestone (

Everyday acid and base reactions. Calcium carbonate and rocks. Limestone is also largely composed of calcium carbonate. Bath Stone (Greater Oolite) is. - ppt download

Effect of simulated acid rain on the stability of calcium carbonate immobilized by microbial carbonate precipitation - ScienceDirect
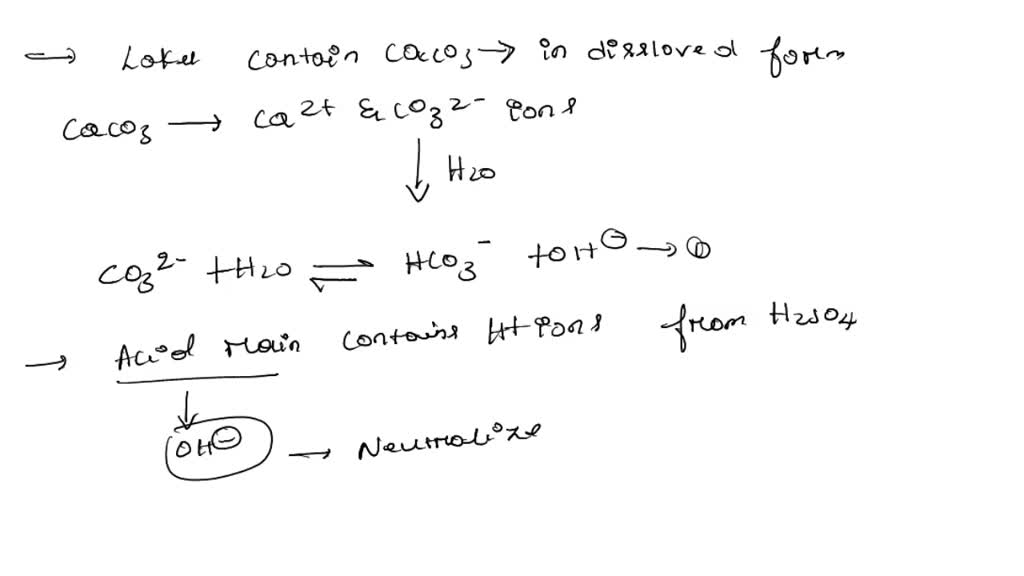
SOLVED: Lakes have a natural buffering capacity, especially in regions where limestone gives rise to dissolved calcium carbonate. Write an equation for the effect of a small amount of acid rain containing

Acid Deposition Lake Barkevatn in Norway used to have healthy stocks of trout and perch. As a result of acid rain, the trout stock died out in the mid-1970s. - ppt download

Where does our drinking water come from? This means the water we drink has run through and across rocks. - ppt download

Toward the Mechanistic Understanding of the Additives' Role on Ammonium Nitrate Decomposition: Calcium Carbonate and Calcium Sulfate as Case Studies | ACS Omega
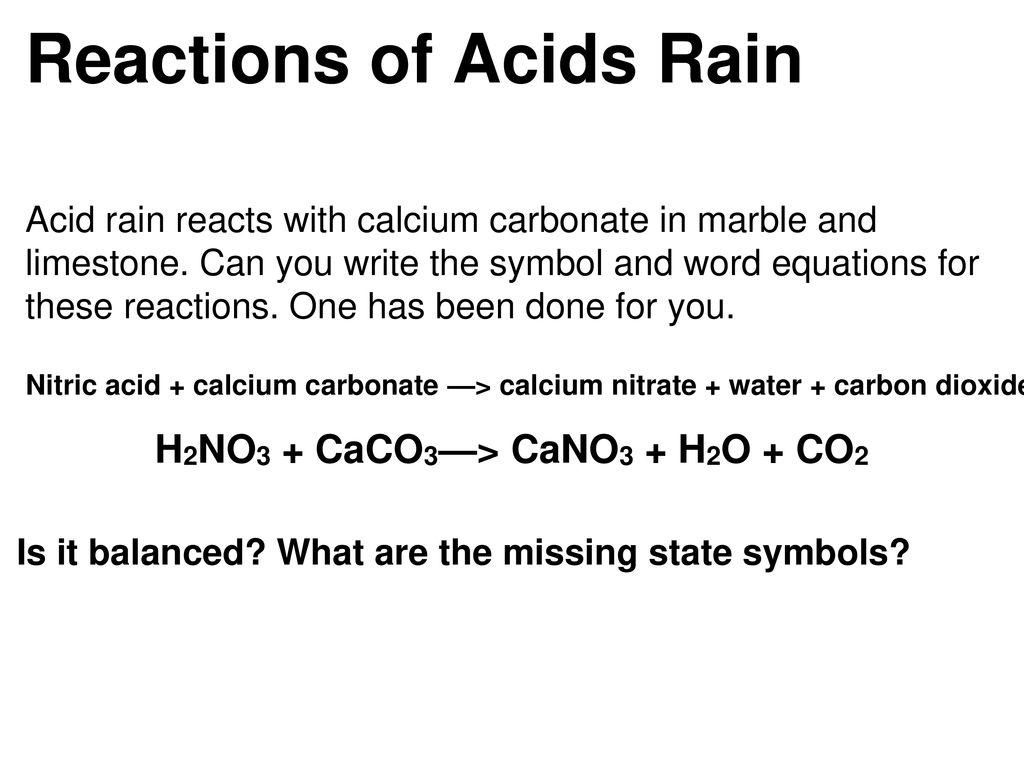
CWK CWK Acid Rain State the adverse effect of these common pollutants on buildings and why these pollutants are of global concern Relate the effects. - ppt download
How does acid rain, formed when sulphur dioxide and oxides of nitrogen dissolve in rain water, destroy buildings and statues, especially those made from limestone (a form of calcium carbonate)? - Quora