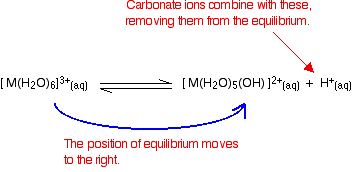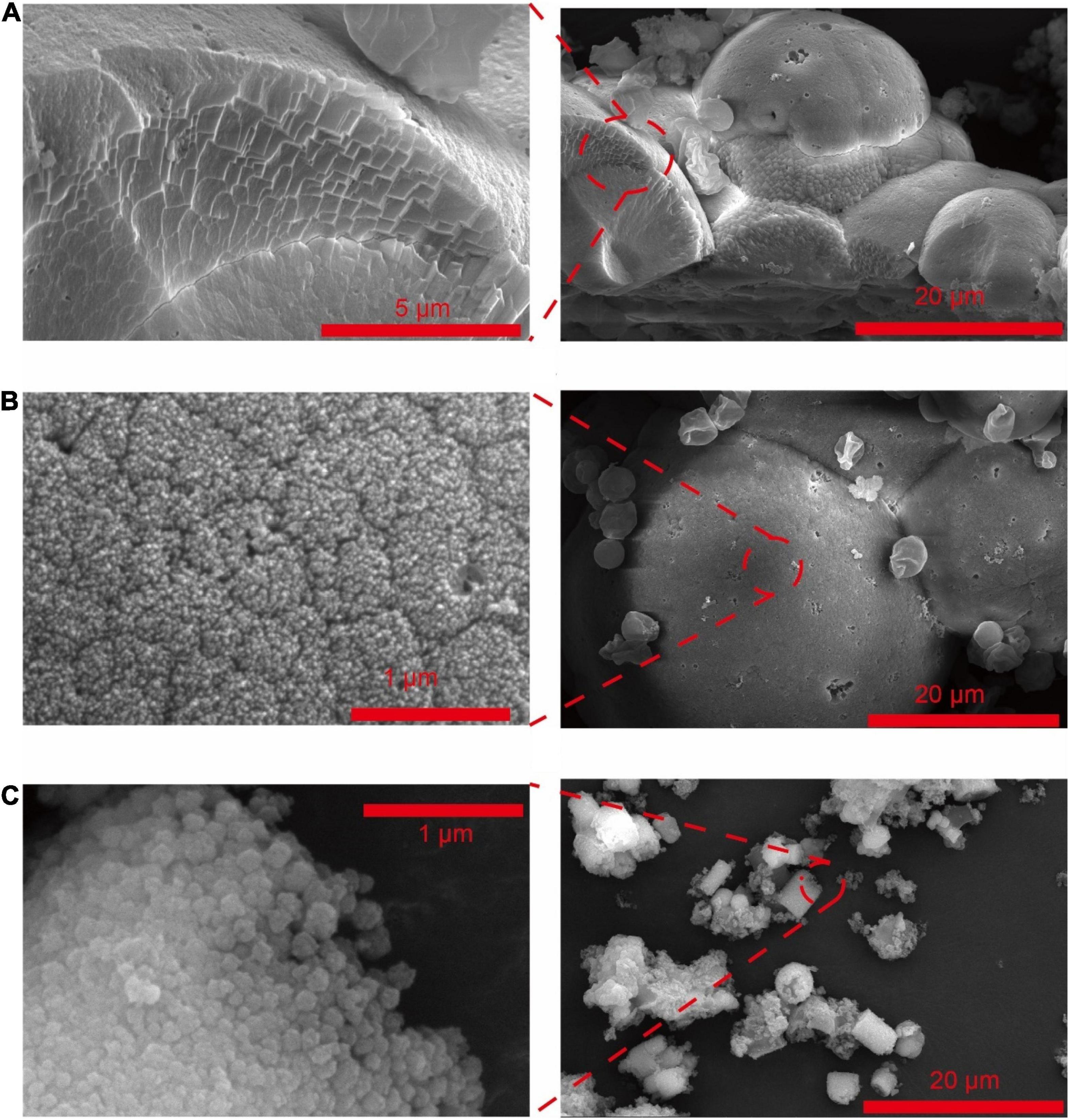
Frontiers | New Insights Into Microbial Induced Calcium Carbonate Precipitation Using Saccharomyces cerevisiae

Write the balanced chemical equations for the following reactions.(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water(b) Zinc + Silver nitrate → Zinc nitrate + Silver(c) Aluminium + Copper chloride

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride

Sustainable iron production from mineral iron carbonate and hydrogen - Green Chemistry (RSC Publishing) DOI:10.1039/C6GC02160C
What is the balanced net-ionic equation for the gas producing reaction between hydrobromic acid and solid calcium carbonate? - Quora
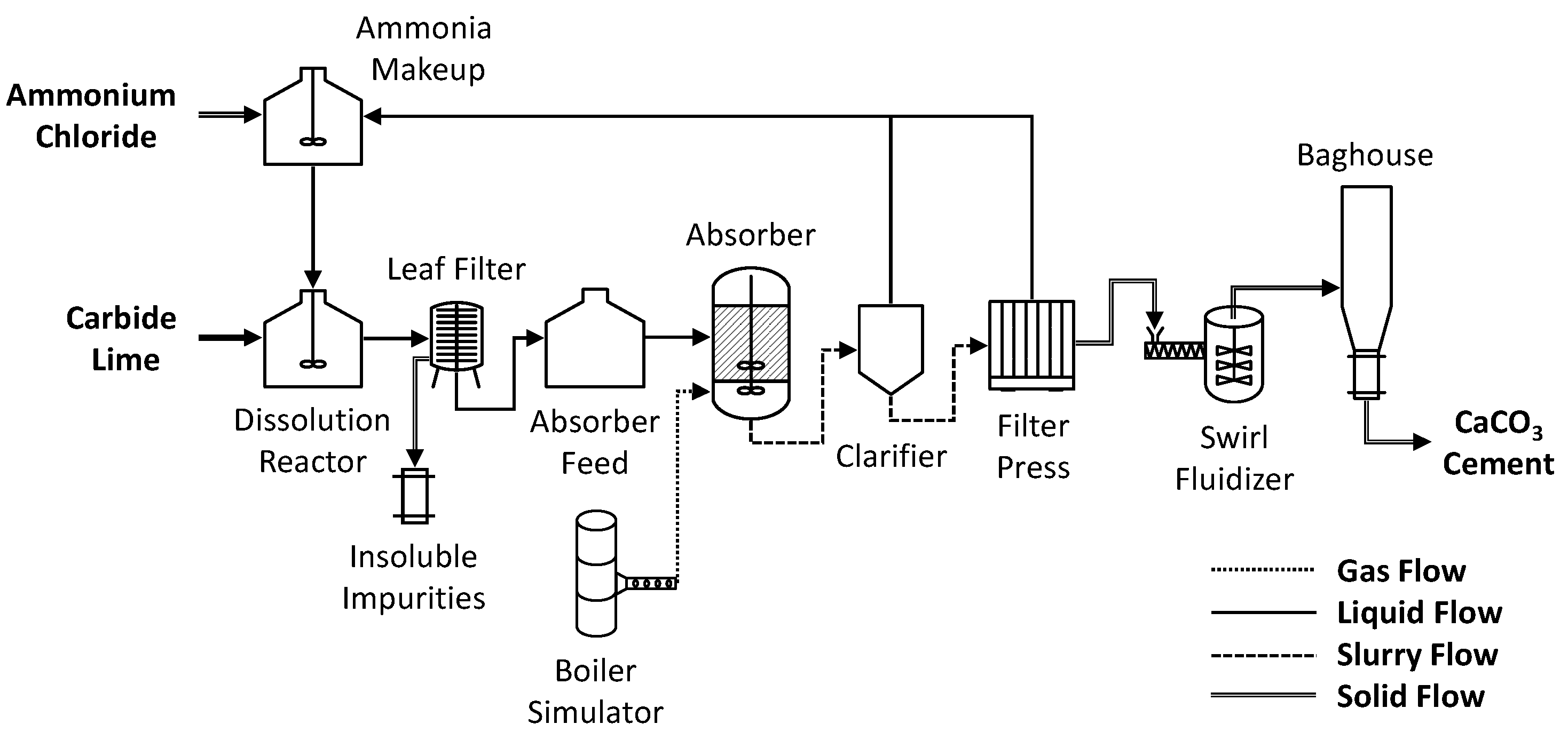
Materials | Free Full-Text | Calcium Carbonate Cement: A Carbon Capture, Utilization, and Storage (CCUS) Technique

Toward the Mechanistic Understanding of the Additives' Role on Ammonium Nitrate Decomposition: Calcium Carbonate and Calcium Sulfate as Case Studies | ACS Omega

Iron Calcium Carbonate Instability: Structural Modification of Siderite Corrosion Films | ACS Applied Materials & Interfaces

Recovery of iron oxide and calcium chloride from an iron-rich chloride waste using calcium carbonate | SpringerLink

Reaction steps involved in the formation of the CaCO3 sol from calcium... | Download Scientific Diagram
Write symbolic representation for the following word equations and balance them : (a) Calcium carbonate → Calcium oxide + Carbon dioxide - Sarthaks eConnect | Largest Online Education Community

LR whiteboard practice. NH 3 + O 2 NO + H 2 O. In an experiment, 3.25 g of NH 3 are allowed to react with 3.50 g of O 2. a. Which reactant is the limiting. - ppt download
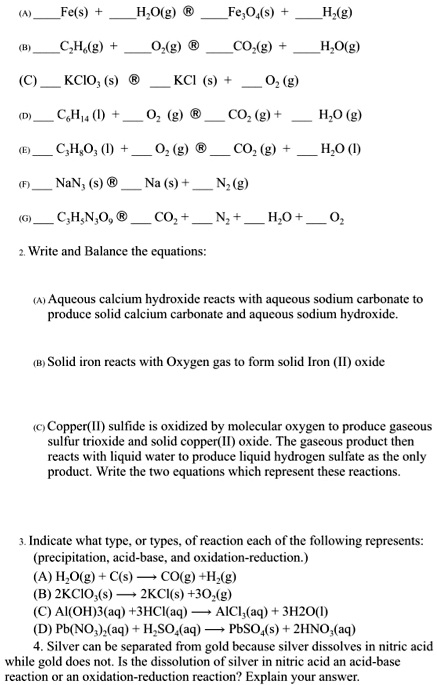
SOLVED: Fe(s) H,O(g) Fe,O(s) H,(g) CH(g) 0,(g) @ CO-(g) H,O(g) KCIO; (s) KCI (s) 0z (2) C;l,a () COz HO (g) CSH;O; (I) 0z (g) COz H,O () NaN; (s) @ (S)t

Molecules | Free Full-Text | Formation of Copper Oxide Nanotextures on Porous Calcium Carbonate Templates for Water Treatment
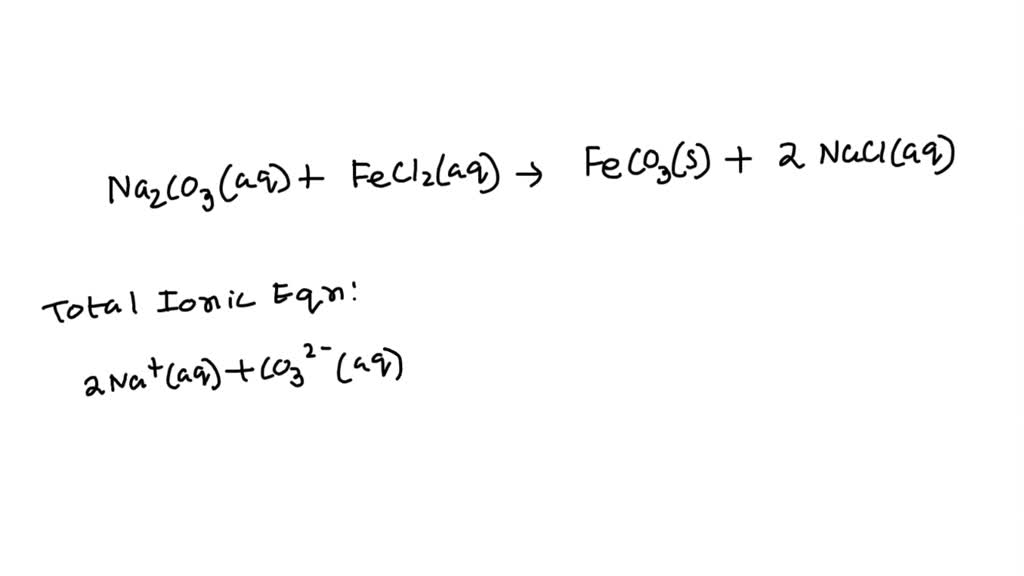
SOLVED: Write the net ionic equation for the reaction of Sodium carbonate with Iron (II) chloride. (what in the equation are electrolytes?
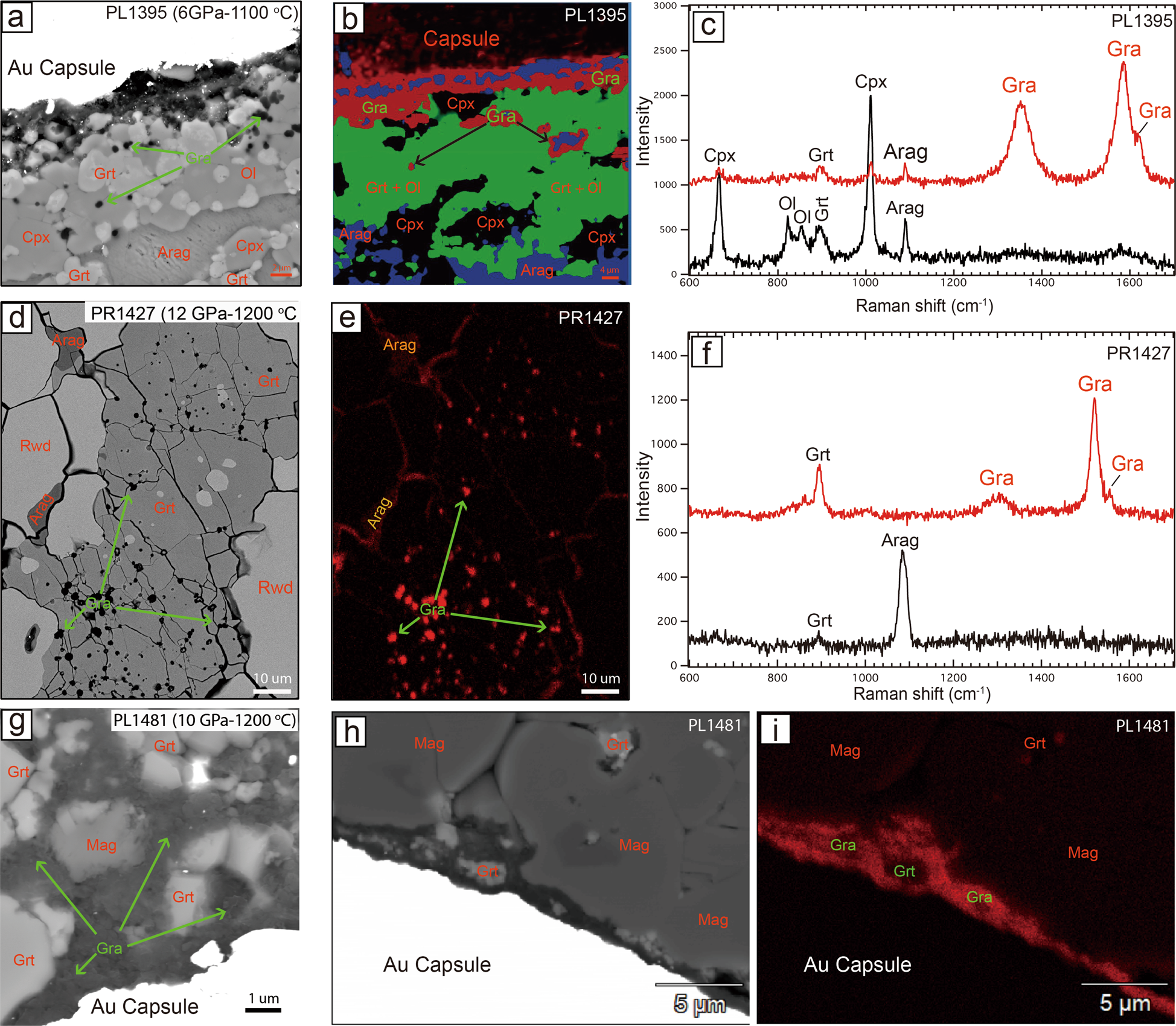
Recycled calcium carbonate is an efficient oxidation agent under deep upper mantle conditions | Communications Earth & Environment