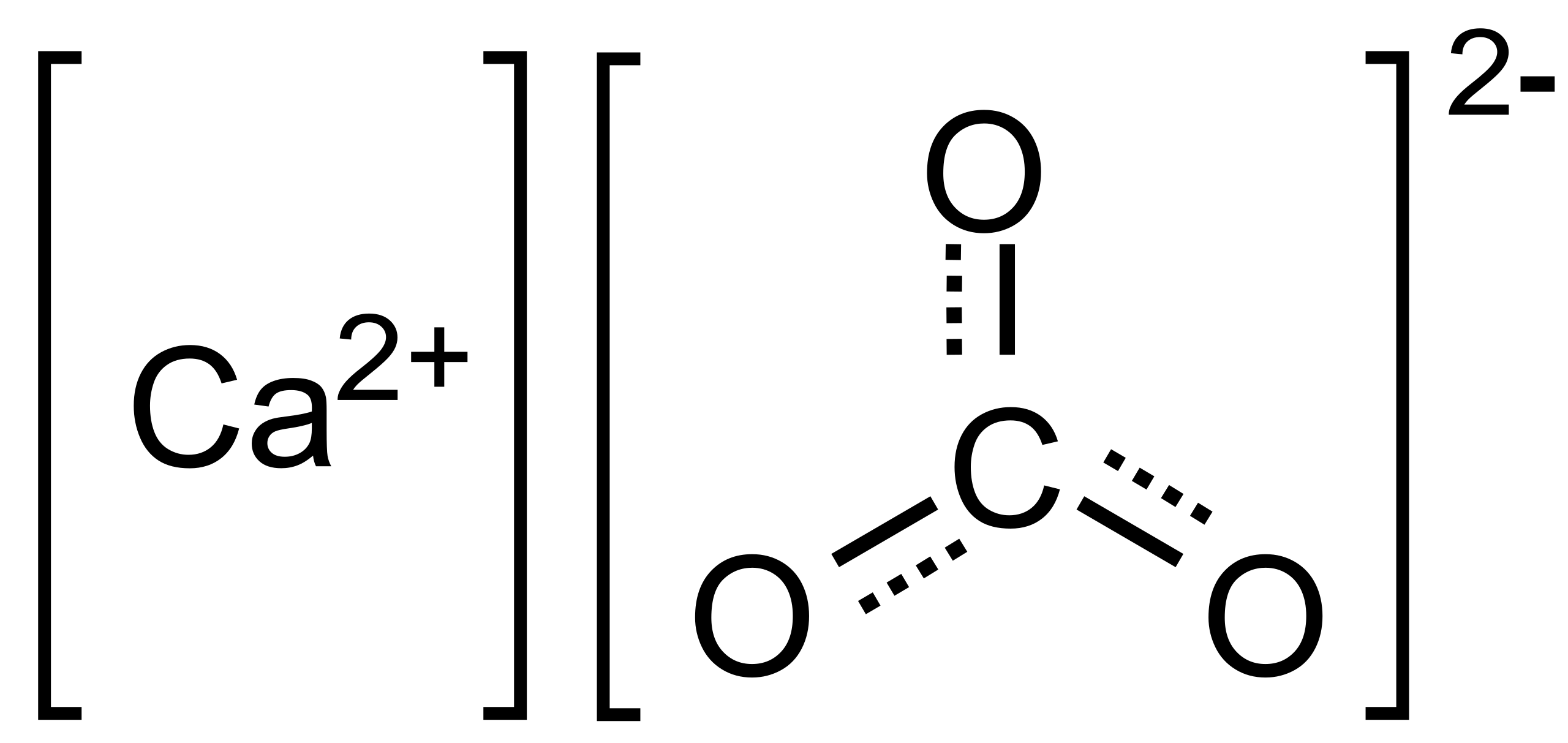
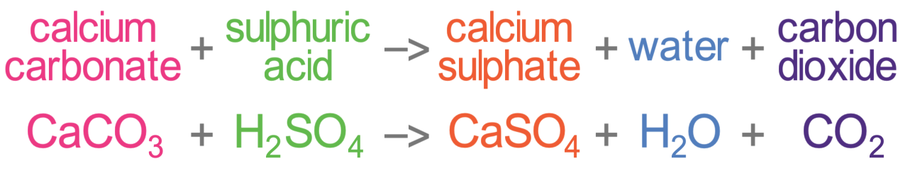
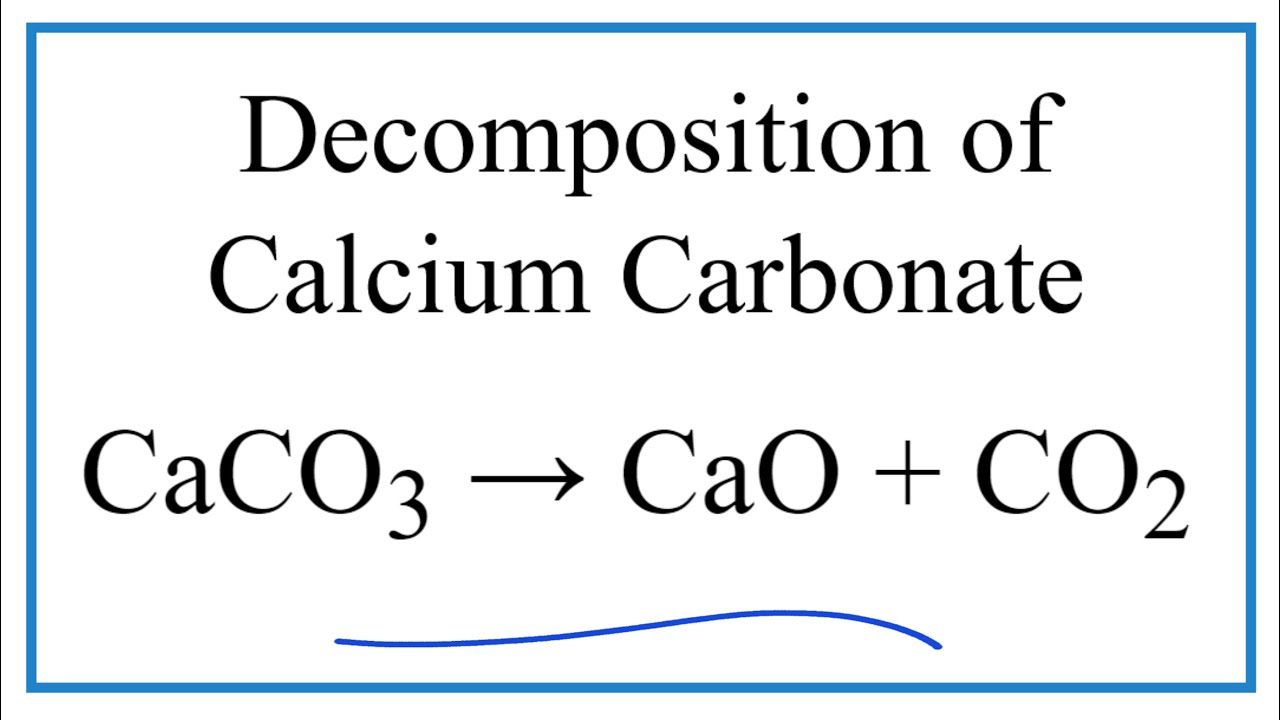
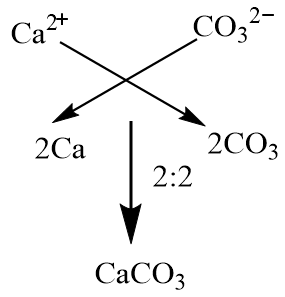
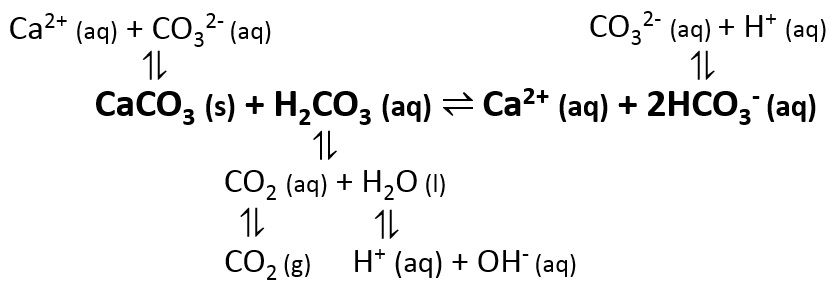How to Balance Ca(HCO3)2 = CaCO3 + CO2 + H2O (Decomposition of Calcium hydrogen carbonate) - YouTube

SOLVED: EDTA solutions of a known concentration are used to determine water hardness These solutions are standardized using oven-dried calcium carbonate (CaCO3). Calcium carbonate reacts with EDTA according to the equation: Ca2+
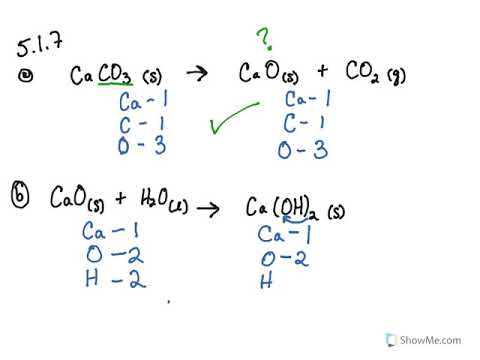
Write balanced chemical equation for the following processes: (a) heating calcium in oxygen (b) heating calcium carbonate - Sarthaks eConnect | Largest Online Education Community

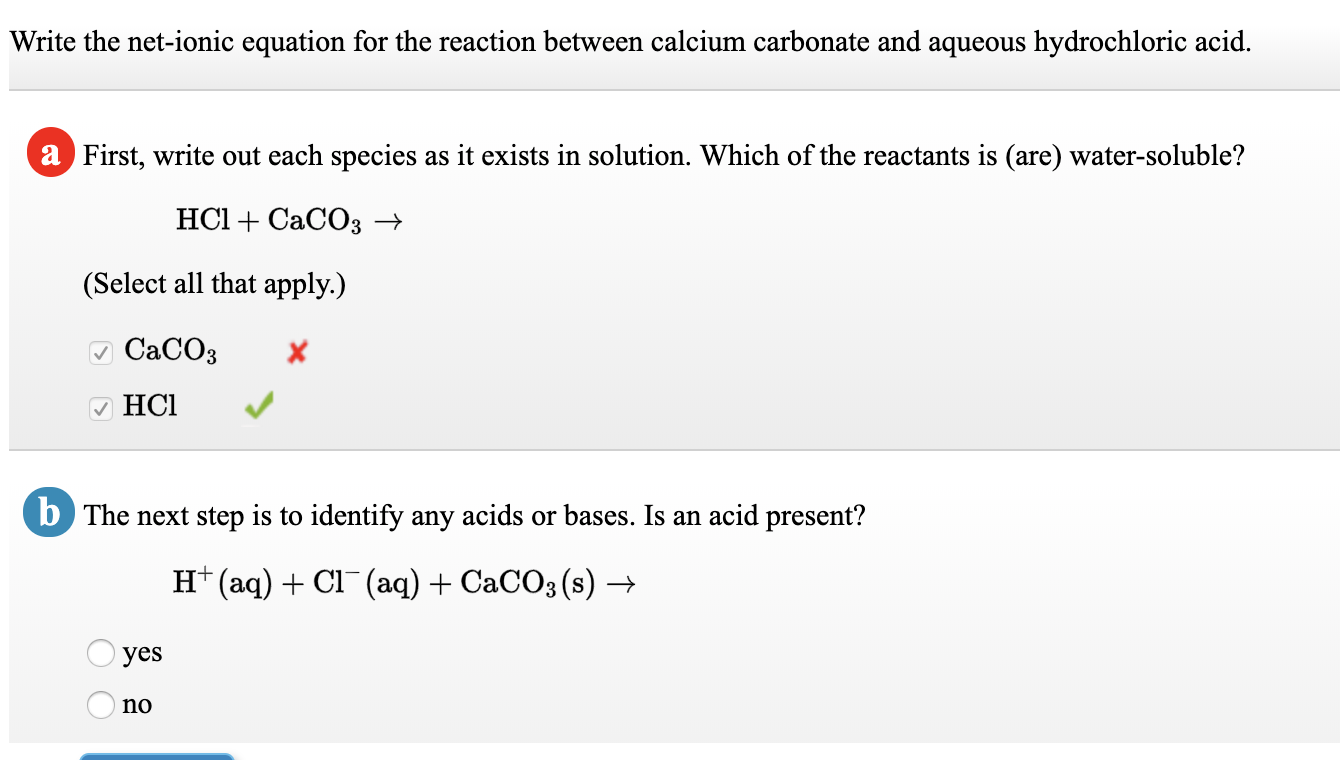
Question Video: Calculating the Average Rate of Reaction of Hydrochloric Acid with Calcium Carbonate | Nagwa