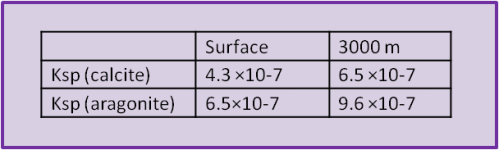
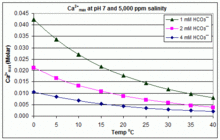
Solubility product (log_KSP) of various Ca-carbonates for temperatures... | Download Scientific Diagram
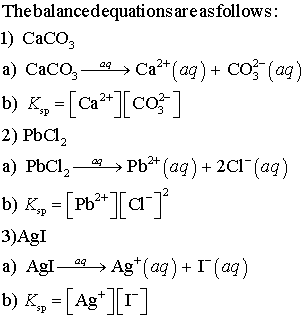
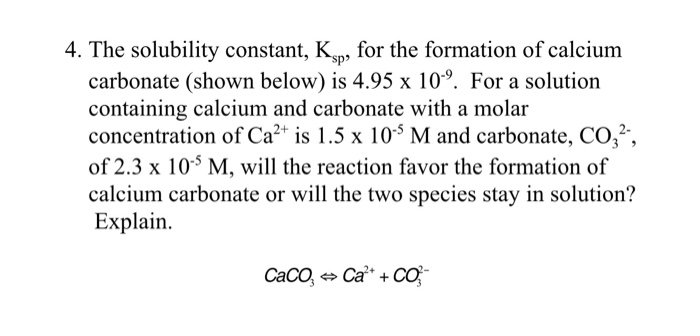
Write a balanced equation for the dissolution of CaCO3.Write an expression for Ksp for the dissolution - Home Work Help - Learn CBSE Forum
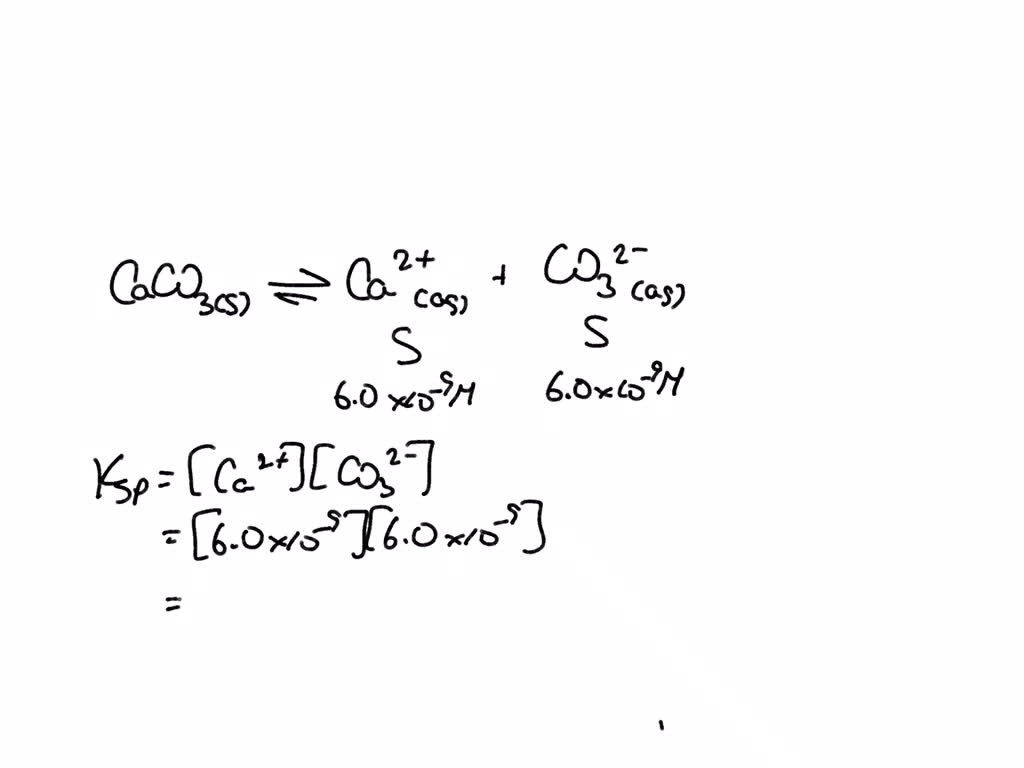
SOLVED: The molar solubility of calcium carbonate (CacO3) in water is 6.0x 10- mol/L Calculate its Ksp 0 7.Jx10-5 64x10-14 0 36* 10-80 0 40x10-5 40*10-15

Ksp for calcium carbonate is 3 x 10^(-9). If you mix together 100 mL of 0.01 M calcium chloride with 100 mL of 10^(-5) M solution of potassium carbonate, will a precipitate form? | Homework.Study.com
The values of Ksp of CaCO3 and CaC2O4 are 4.7 x 10^-9 and 1.3 x 10^-9 respectively at 25°C. - Sarthaks eConnect | Largest Online Education Community

The solubility of calcium carbonate MW=100 09 is 1 3×10 2 g 1000 mL of solution What is the Ksp for - YouTube

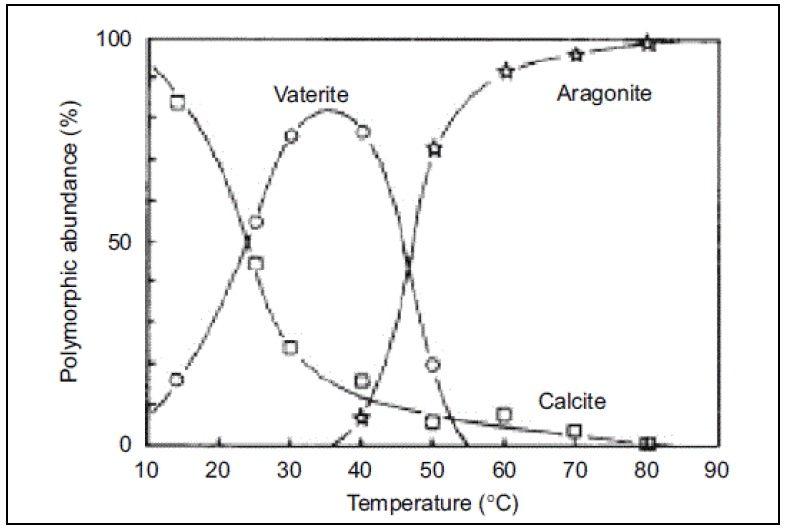
Factors controlling and influencing polymorphism, morphology and size of calcium carbonate synthesized through the carbonation route: A review - ScienceDirect
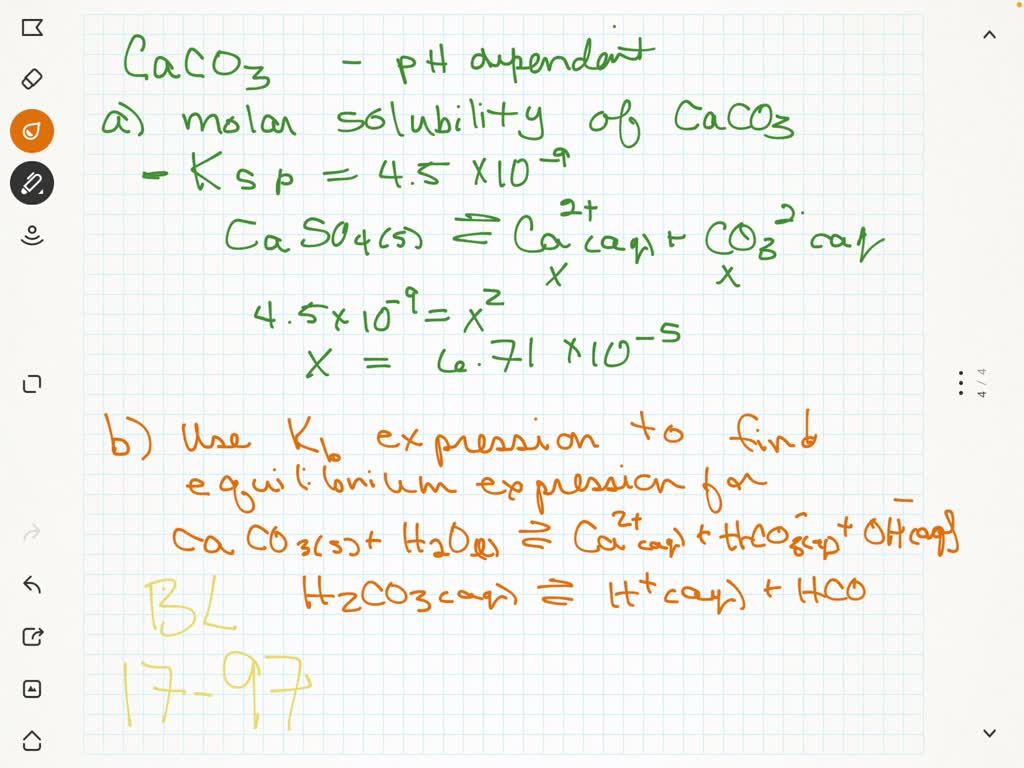
SOLVED:The solubility of CaCO3 is pH dependent. (a) Calculate the molar solubility of CaCO3(Ks p=4.5 ×10^-9) neglecting the acid-base character of the carbonate ion. (b) Use the Kb expression for the CO3^2-
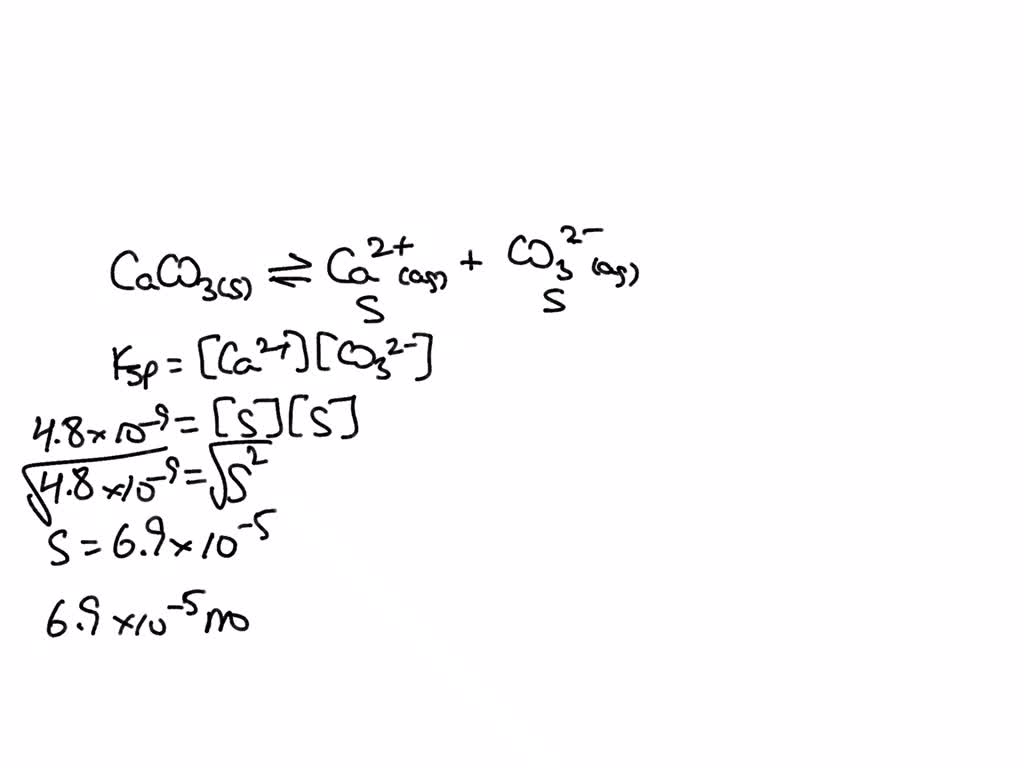
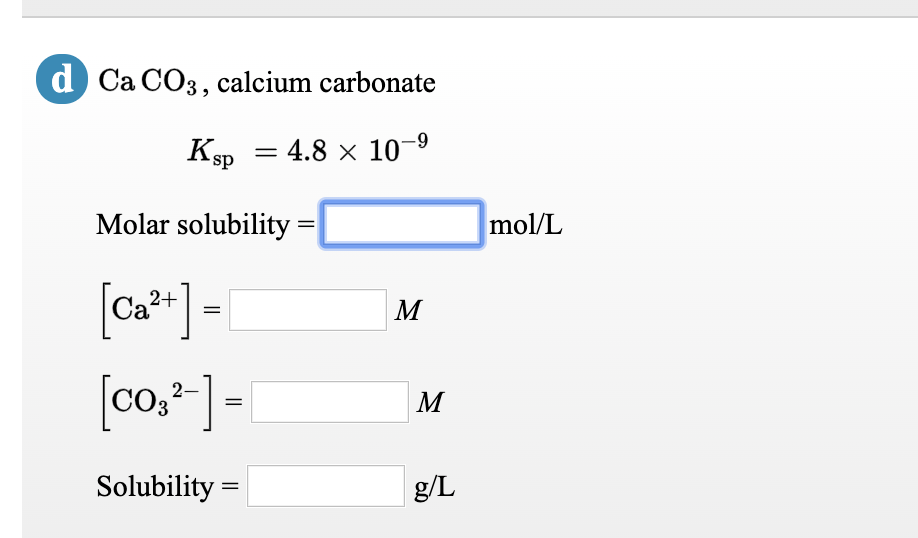
SOLVED: Calculate the solubility of calcium carbonate (CaCO3) in g / L. (Kps, CaCO3 = 4.8x10 ^ -9) (Mm, CaCO3 = 100.9 g / mol)A) 4.8x10^-10B) 6.9x10^-5C) 4.8x10^-9D) 6.9x10^-3

The values of Ksp of CaCO3 and CaC2O4 are 4.7 × 10^-9 and 1.3 × 10^-9 respectively at 25^∘C . If the mixture of these two is washed with water, what is
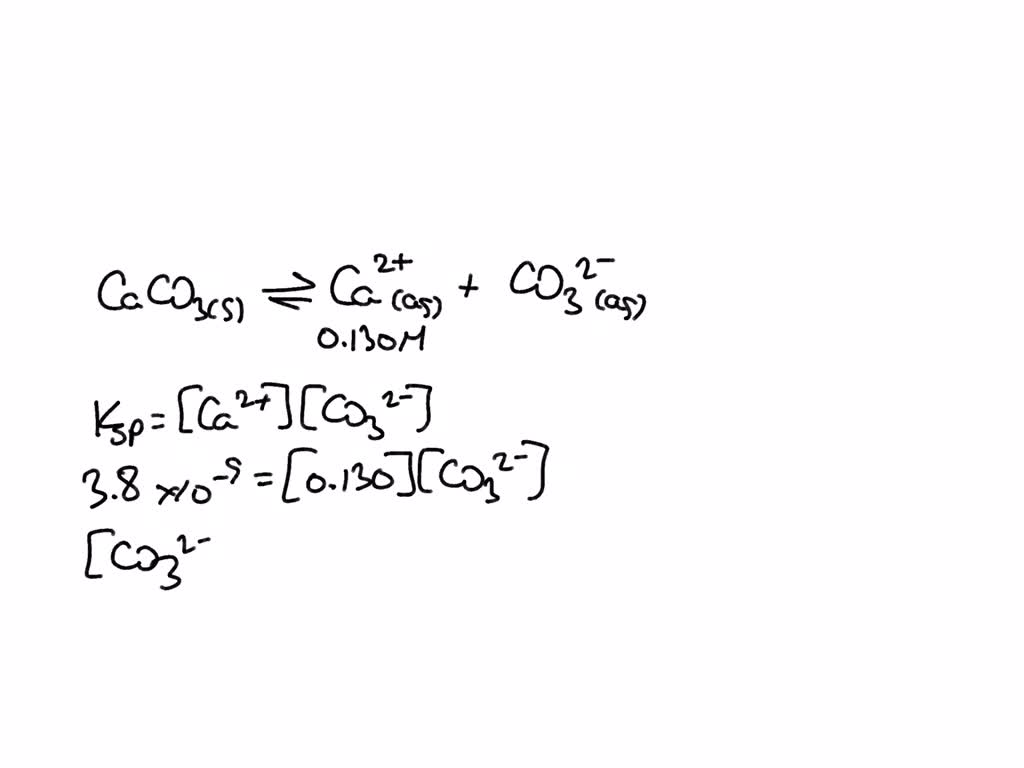
SOLVED: Is calcium carbonate more or less soluble in a solution contain 0.25 M potassium carbonate or solution containing 0.25 M calcium nitrate? Explain using equilibrium principles: (Ksp calcium carbonate 4.8E-9 (Hint: