calcium carbonate caco3 molar mass 100 g mol is an acid used to neutralize extra acid in the stomach jose is - Brainly.ph
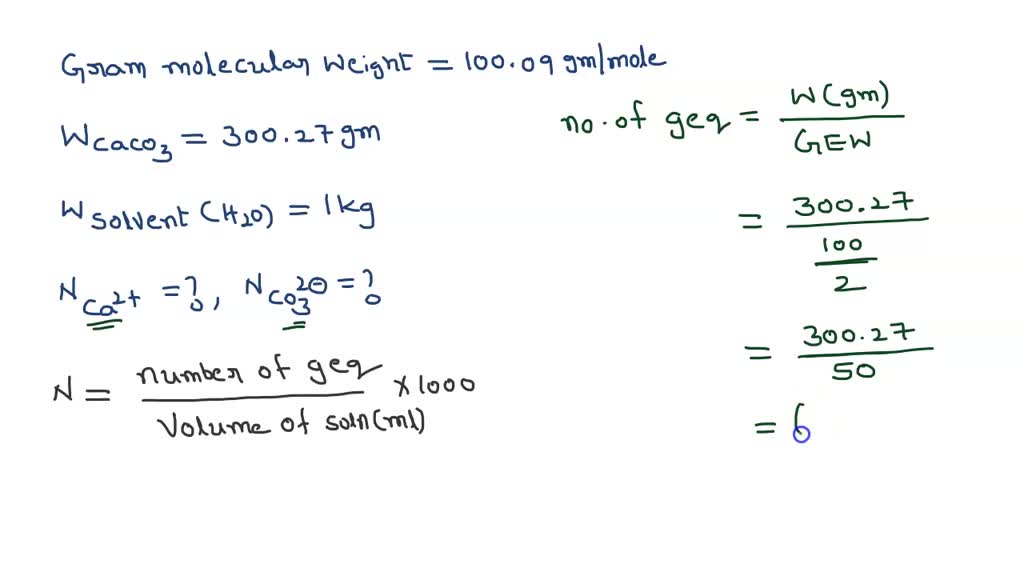
SOLVED: 3. The molecular mass of CaCO3 is 100.09 g/mole. If 300.27 g of CaCO3 is dissolved in 1kg of pure water, what is the normality (in eq/kg of Ca2+ and CO3

Molar mass CaCO3|Calculate molecular weight Calcium carbonate|Calcium carbonate Molar mass - YouTube

Calculate the moles of carbon dioxide that are released when 44.7 g of calcium carbonate (molar mass = 100.0 - Brainly.in
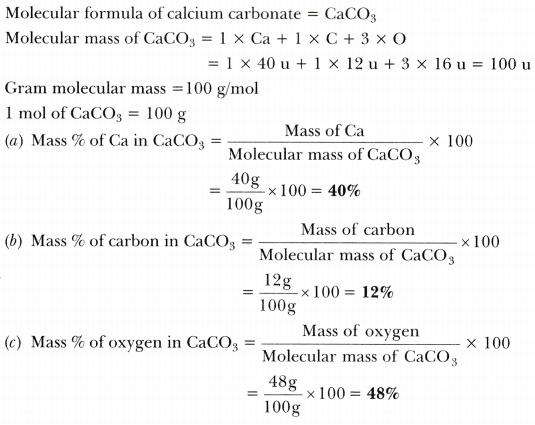
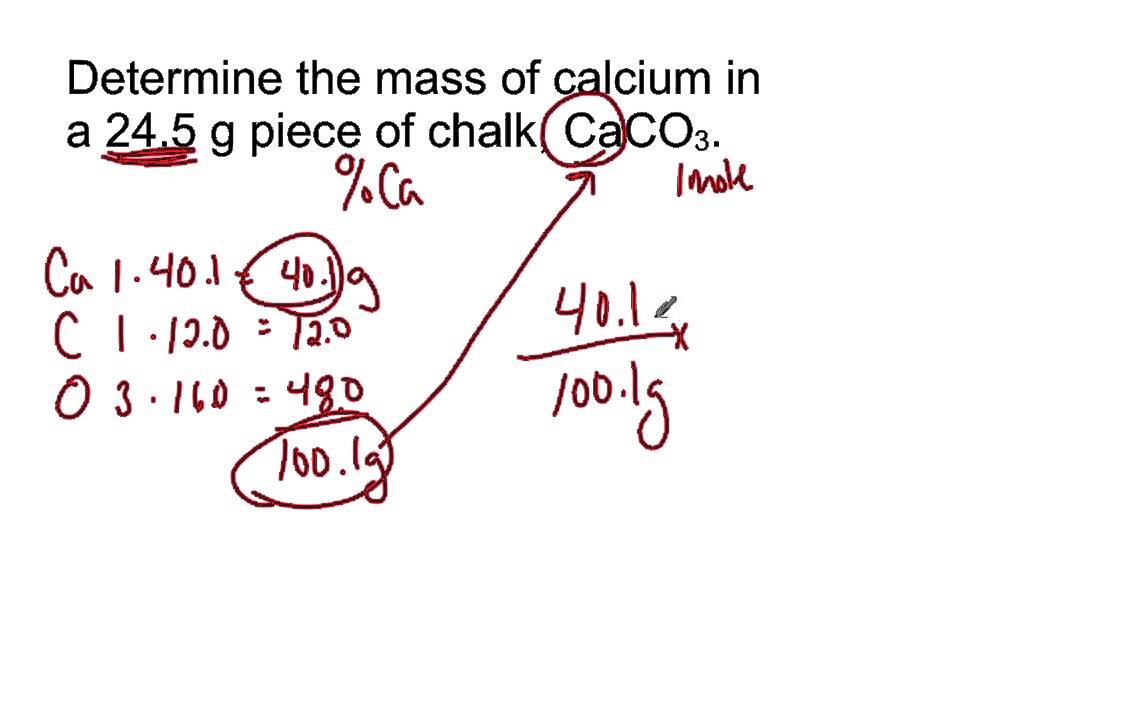
Calculate the mass per cent of each element present in the molecule of calcium carbonate - CBSE Class 9 Science - Learn CBSE Forum
If 220 grams of calcium oxide (CaO) reacts with 50L of carbon dioxide (CO2), what mass of calcium carbonate (CaCO3) is produced? - Quora
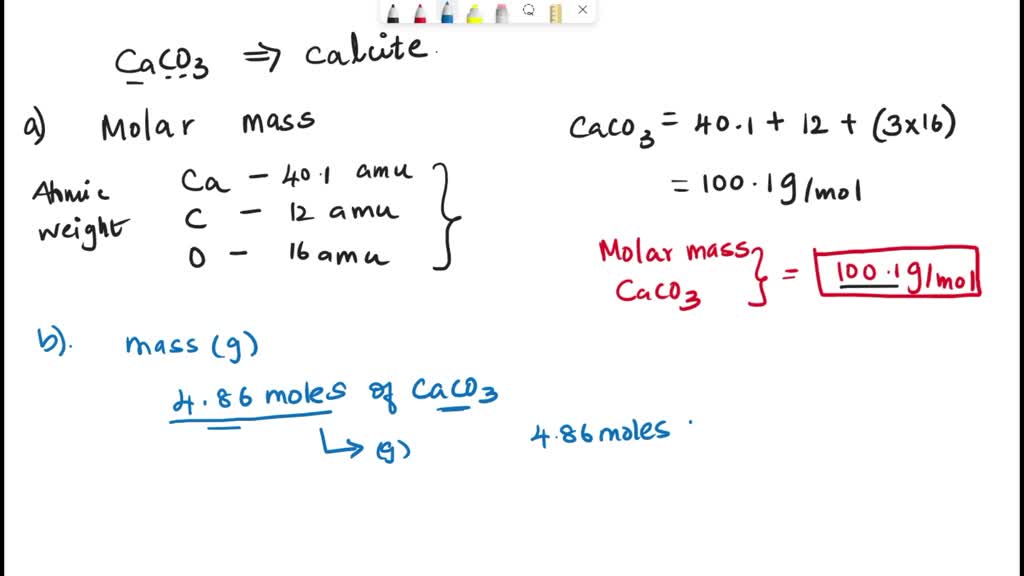
SOLVED: Calcium carbonate (CaCO;), also called calcite: Calculate the molar mass of caleium carbonate. A certain sample of calcium carbonate contains 4.86 moles What is the mass in this sample? grams What
calcium carbonate -- Critically Evaluated Thermophysical Property Data from NIST/TRC Web Thermo Tables (WTT)
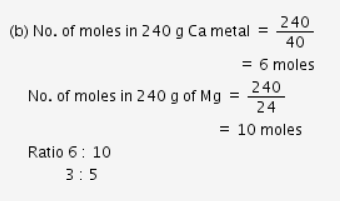
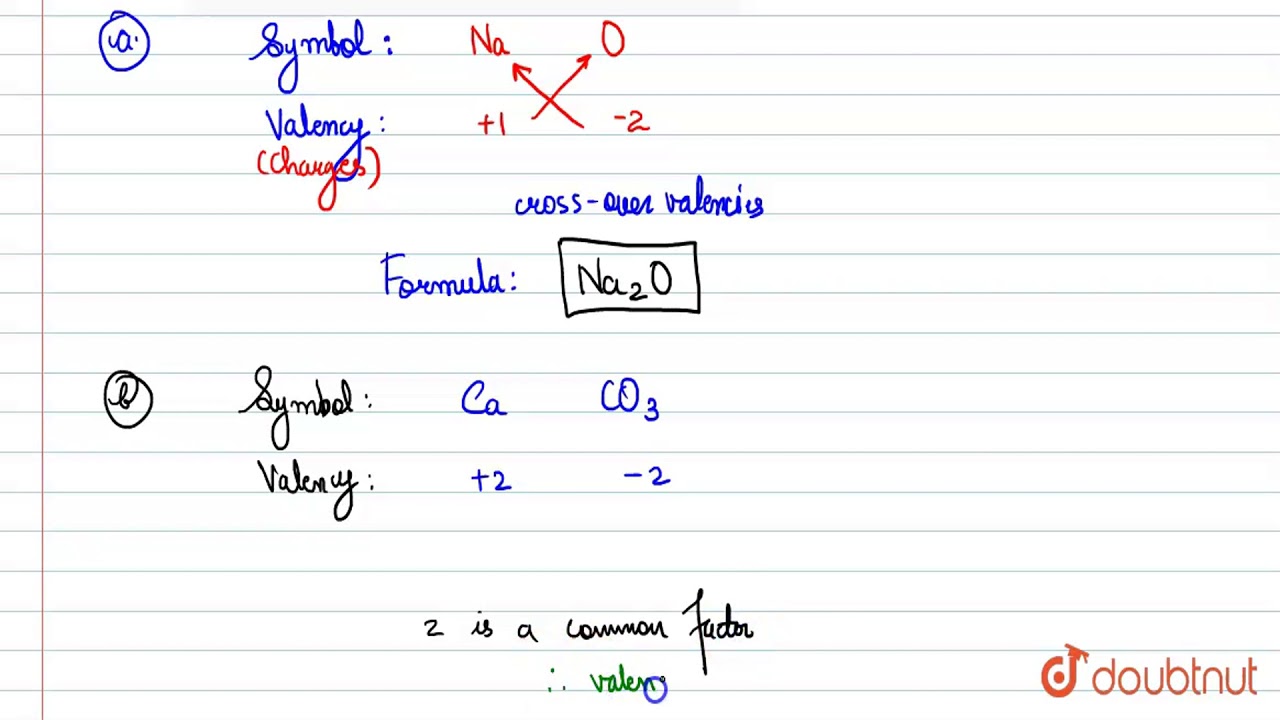
Calculate the molecular mass of CaCO3 (At mass Ca = 40 u, C = 12 u, O = 16 u) - CBSE Class 9 - Learn CBSE Forum