
SciELO - Brazil - Comparative evaluation of the pH of calcium hydroxide powder in contact with carbon dioxide (CO2) Comparative evaluation of the pH of calcium hydroxide powder in contact with carbon

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride
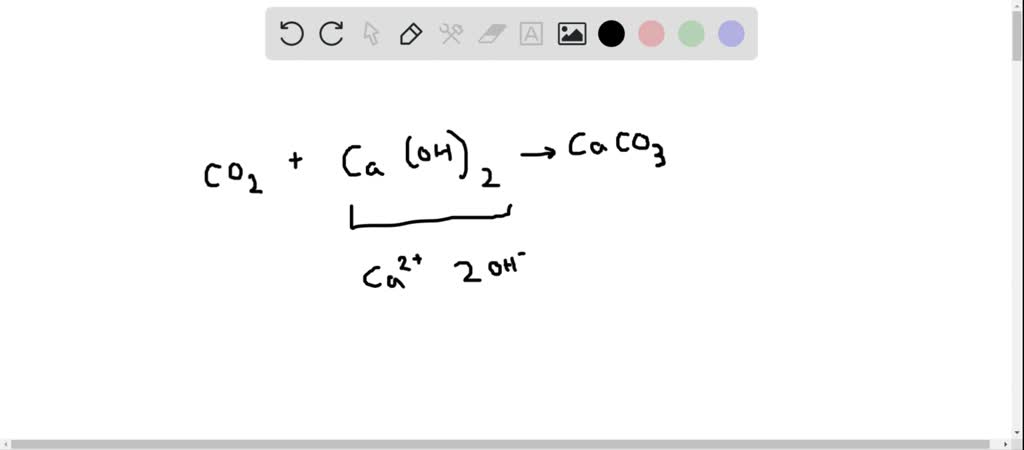
SOLVED:When carbon dioxide is bubbled through a clear calcium hydroxide solution, the solution appears milky. Write an equation for the reaction, and explain how this reaction illustrates that CO2 is an acidic

Optimization of the structural characteristics of CaO and its effective stabilization yield high-capacity CO2 sorbents | Nature Communications
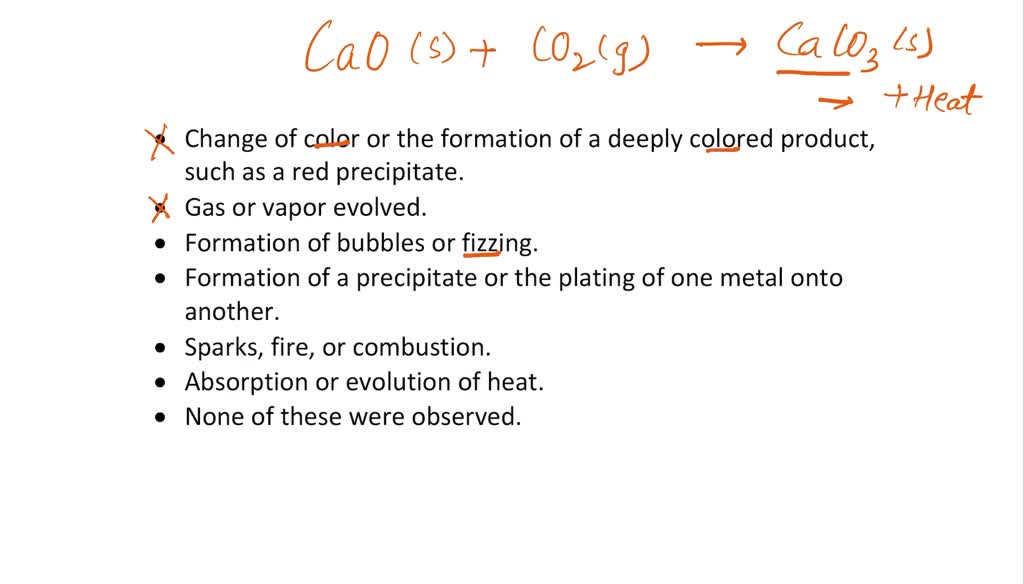
SOLVED: Reaction of calcium oxide and carbon dioxide: Watch the video for this experiment and list each of the changes you observed. Change of color or the formation of deeply colored product

When excess of carbon dioxide is passed through lime water, the milkiness first formed disappears due to:
Write the balanced chemical equations for the following reactions: i. Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water - Sarthaks eConnect | Largest Online Education Community

CO2 capture and ions removal through reaction with potassium hydroxide in desalination reject brine: Statistical optimization - ScienceDirect
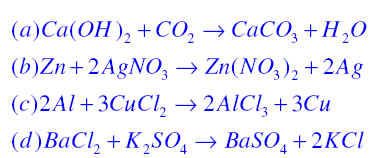
Write the balanced chemical equations for the following reactions. (a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water | Pushpender86's Blog

Q3 Give a balanced equation for the following conversions In one or two steps 1 Coke to water gas 2 ...



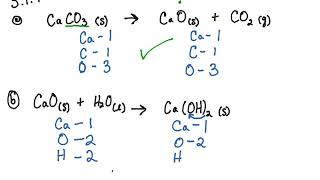









![SQP] A clear solution of slaked lime is made by dissolving Ca(OH)2 SQP] A clear solution of slaked lime is made by dissolving Ca(OH)2](https://d1avenlh0i1xmr.cloudfront.net/edaba2bb-20df-4980-9a2a-d6fc77a0b818/reaction-of-slaked-lime-with-co2---teachoo.jpg)


