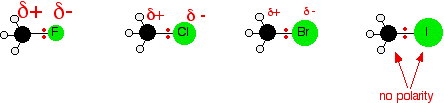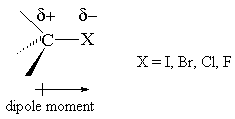Carbon-halogen bond activation by a structurally constrained phosphorus(III) platform | Zhu Group at Xiamen University

Dicationic oligotelluroxane or mononuclear telluronium cation? Elucidation of the true catalytic species and activation mechanism of the benzylic carbon-halogen bond - Chemical Communications (RSC Publishing)

Carbon-halogen bond activation by a structurally constrained phosphorus(III) platform - ScienceDirect
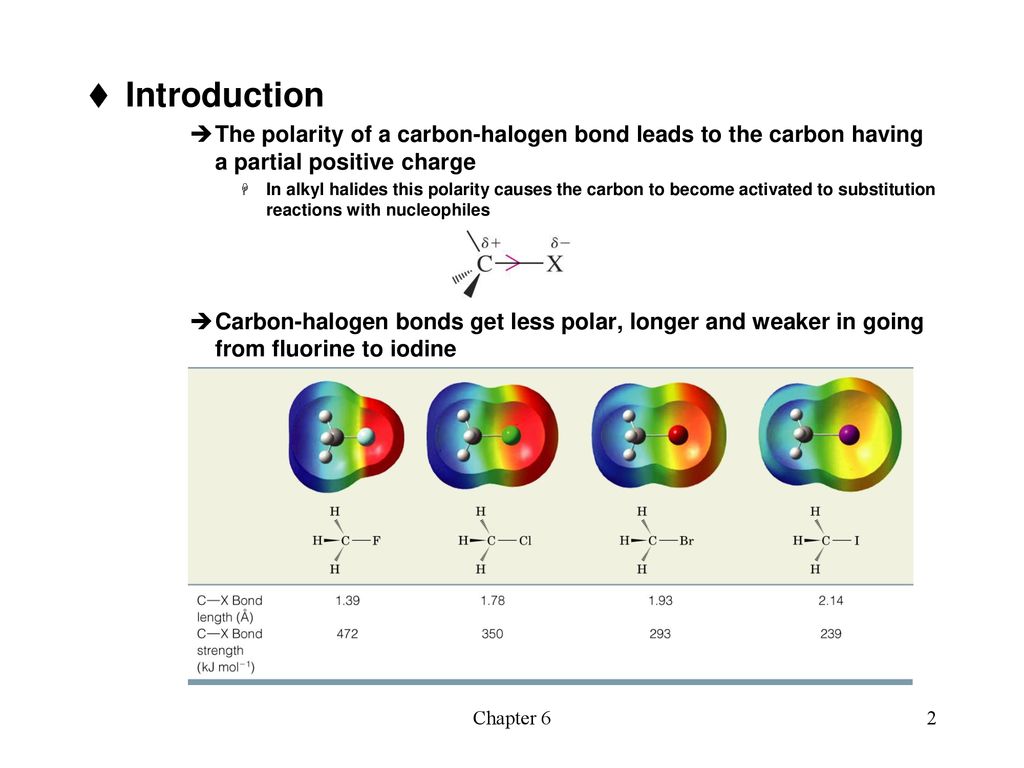
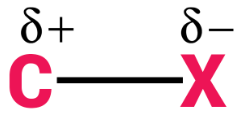
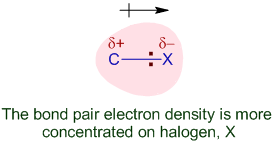
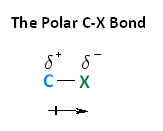
Introduction The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity causes the carbon. - ppt download
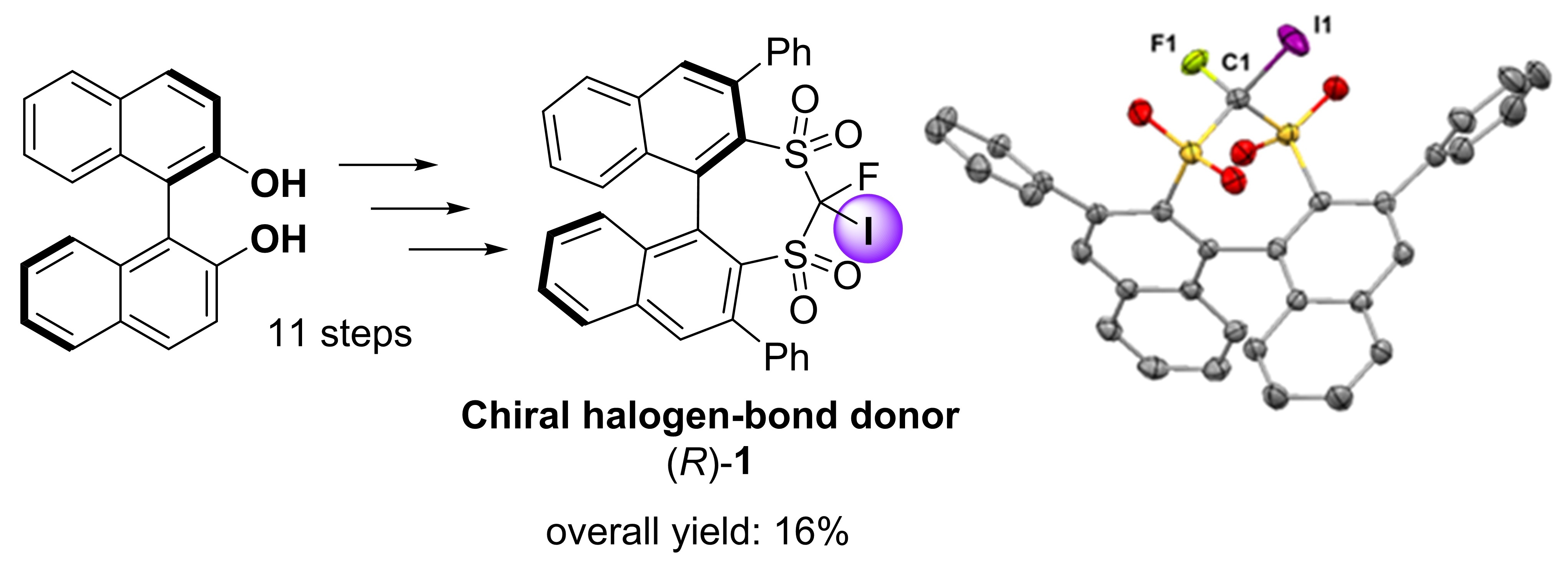
Molecules | Free Full-Text | Design and Synthesis of a Chiral Halogen-Bond Donor with a Sp3-Hybridized Carbon–Iodine Moiety in a Chiral Fluorobissulfonyl Scaffold
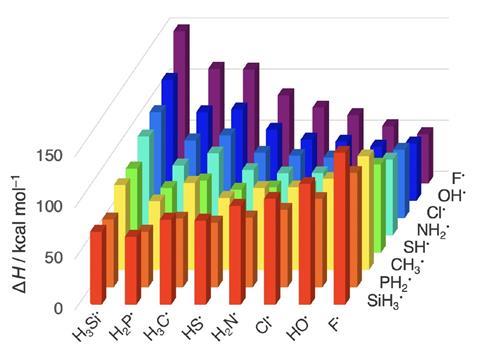
Textbook electronegativity model fails when it comes to carbon–halogen bond strengths | Research | Chemistry World
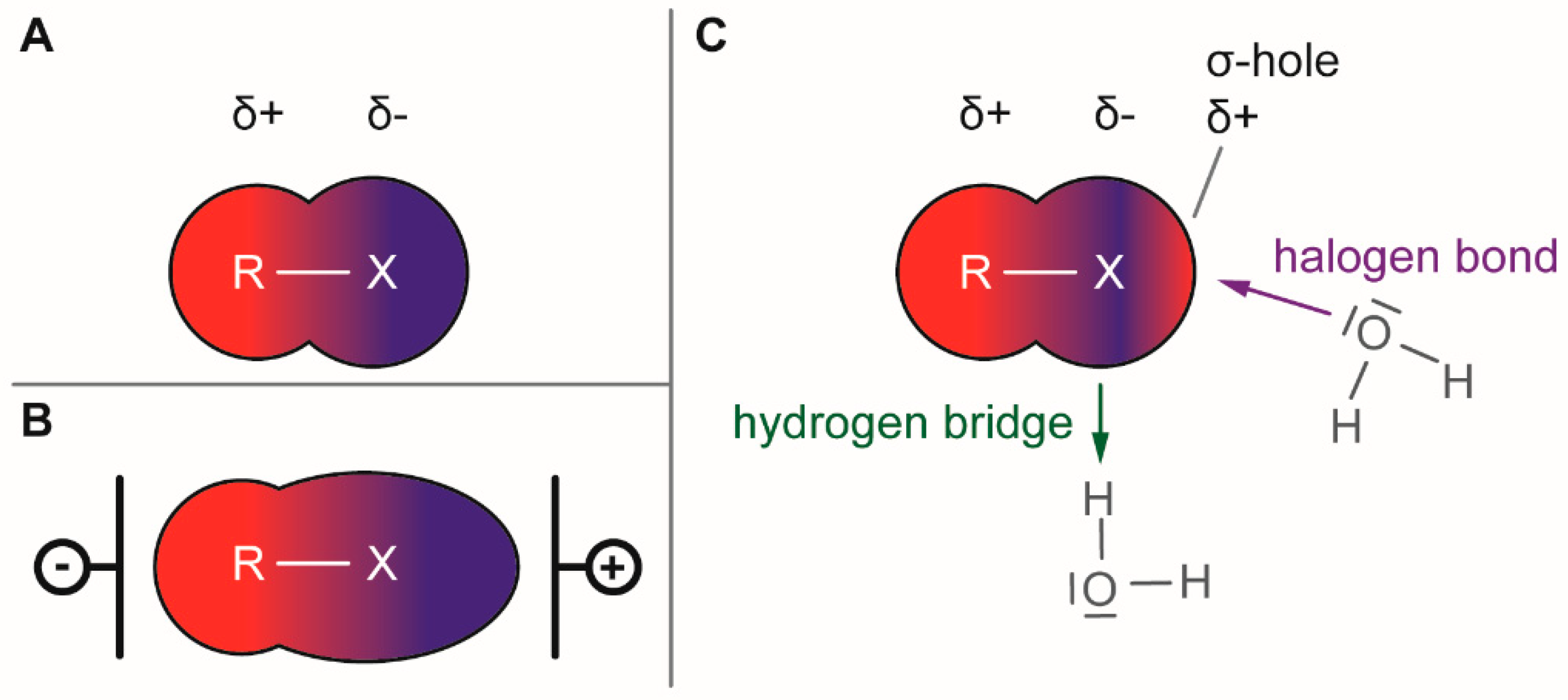
Molecules | Free Full-Text | Halogenating Enzymes for Active Agent Synthesis: First Steps Are Done and Many Have to Follow
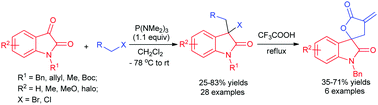
Metal-free formal carbon–halogen bond insertion: facile syntheses of 3-halo 3,3′-disubstituted oxindoles and spirooxindole-γ-butyrolactones - Chemical Communications (RSC Publishing)

Carbon–Halogen Bond Activation by Selenium‐Based Chalcogen Bonding - Wonner - 2017 - Angewandte Chemie International Edition - Wiley Online Library