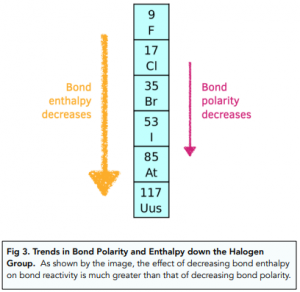
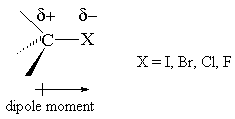Why doesn't the bond polarity of a carbon halogen bond increase from Chlorine~Bromine~Iodine? - Quora

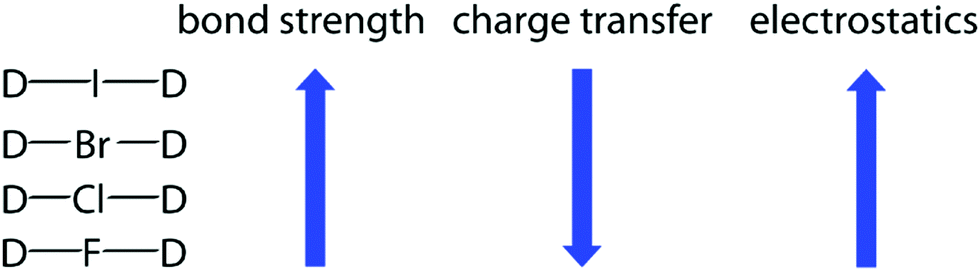
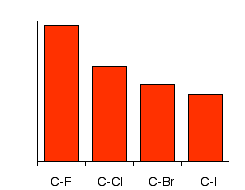
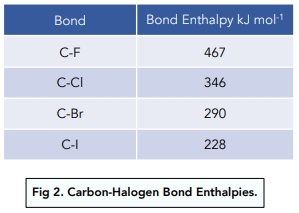
Physical Properties of Haloalkanes 6-1 The bond strength of C-X decreases as the size of X increases. A halogen uses a p orbital to overlap an sp 2 orbital. - ppt download

Reactivity and relative strength of C- Halogen bond in alkyl, allyl, benzyl, vinyl and aryl halides. - YouTube

Physical Properties of Haloalkanes 6-1 The bond strength of C-X decreases as the size of X increases. A halogen uses a p orbital to overlap an sp 2 orbital. - ppt download
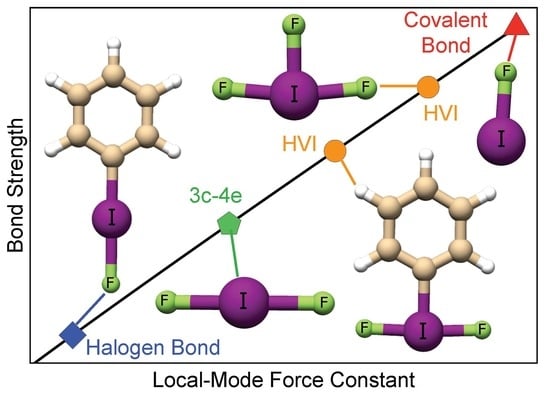
Inorganics | Free Full-Text | A Continuum from Halogen Bonds to Covalent Bonds: Where Do λ3 Iodanes Fit?
![SOLVED: In increasing reactivity in Snl reactions. (Lleast reactive; most reaclive) CH, CF] OMs OMs increasing reactivity in Sn2 reactions. least reactive; most reactive) In increasing carbon-halogen bond strength: wcakest bond; strongest SOLVED: In increasing reactivity in Snl reactions. (Lleast reactive; most reaclive) CH, CF] OMs OMs increasing reactivity in Sn2 reactions. least reactive; most reactive) In increasing carbon-halogen bond strength: wcakest bond; strongest](https://cdn.numerade.com/ask_images/9af6a05d23624cf7ac8e4179310f03f8.jpg)