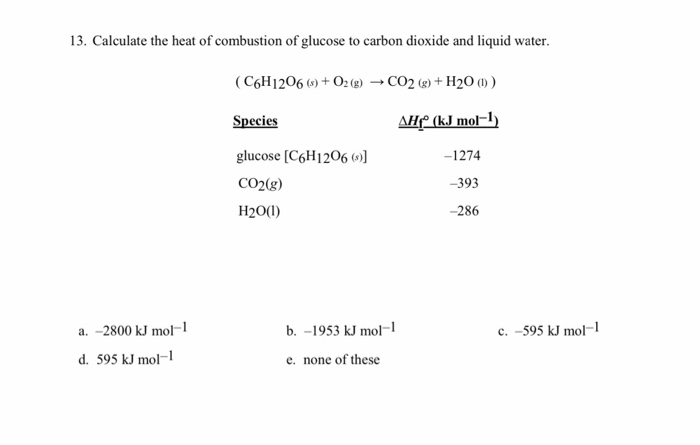
Enthalpy of combustion of carbon to CO2 is - 393.5 KJ/mole. The heat released upon the formation of 35.2g of CO2 from carbon and dioxygen gas is.

Ethylene on combustion gives carbon dioxide and water. Its heat of combustion is 1410.0 kJ . mol^-1 . If the heat of formation of CO2 and H2O are 393.3 kJ and 286.2

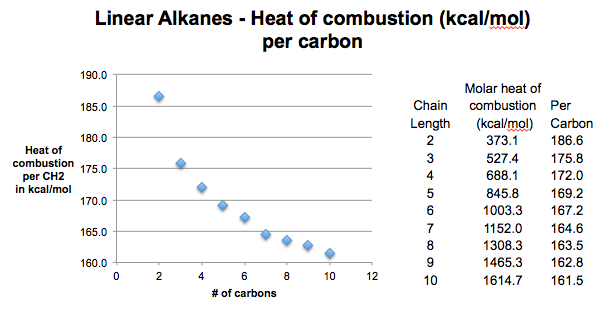
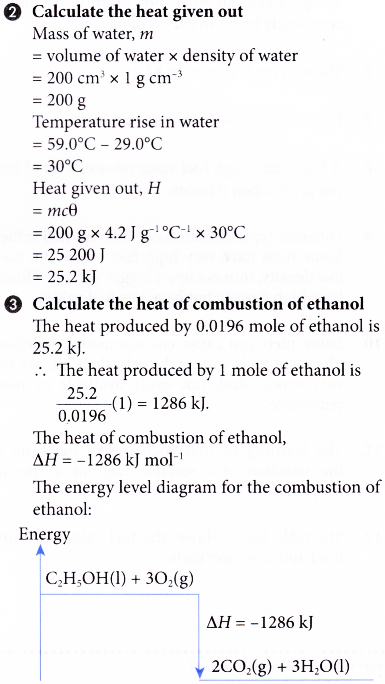
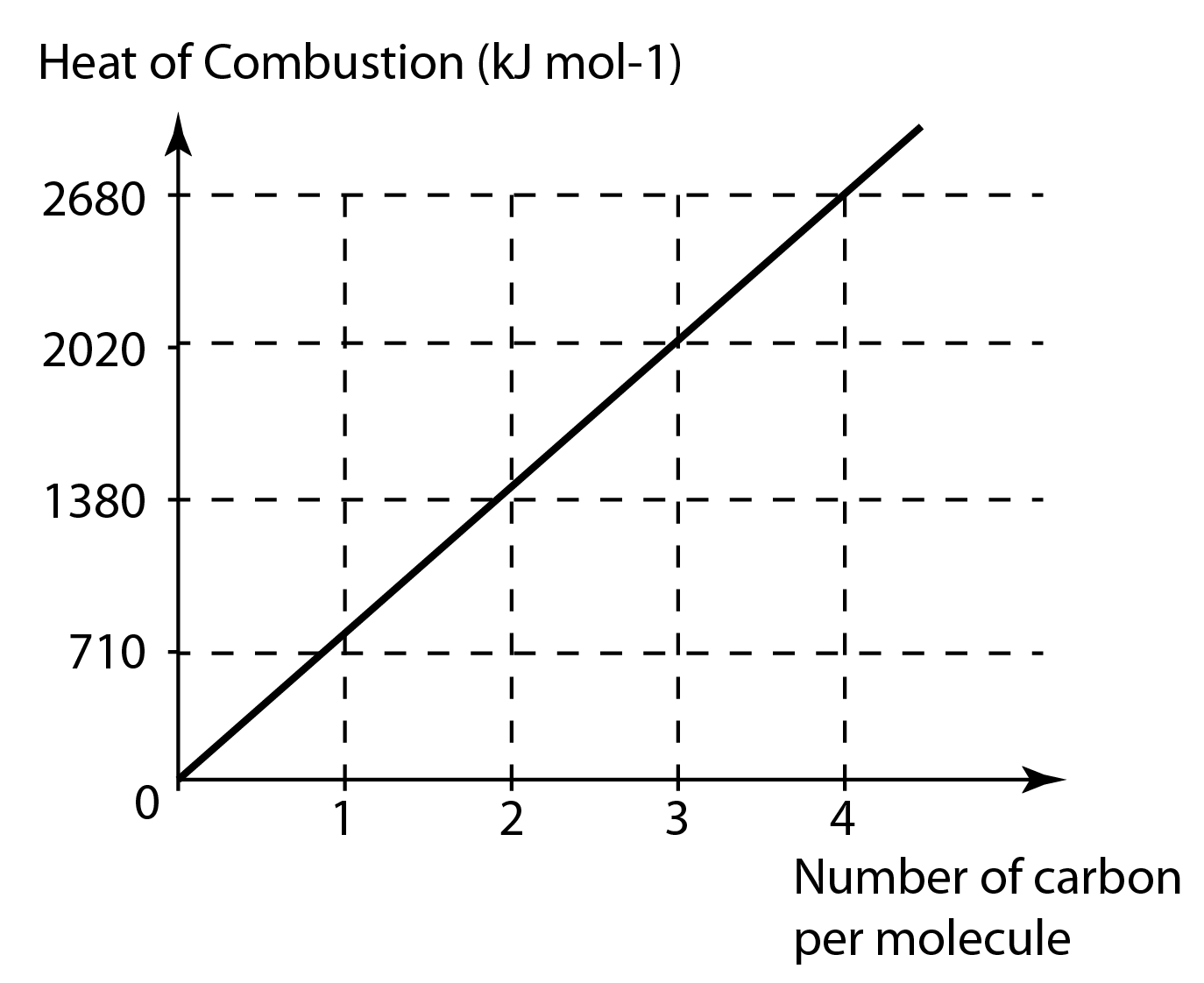
Enthalpy of combustion of alkanes graph vales versus carbon number molecular mass Complete & incomplete combustion of alkanes environmental pollution problems advanced A level organic chemistry revision notes

the heat of combustion of carbon to co2 is -393.5 kj/mol. the heat released upon formation of 35.2 g of co2 - Brainly.in
The heat of combustion of carbon to CO2 is 393.5Kj/mol. The heat rrleased upon formtion of 35.2 g of CO2 from carbn and oxygem gad is
The heat of combustion of carbon to CO2 is 395.5kJ/mol. The heat released upon formation of 35.2 g of CO2 from carbon and oxygen gas is

Calculate the standard heat of formation of carbon disulphide (l). Given that the standard heats of - YouTube
37. the heat of combustion of C,S carbondisulphide 393.3, 293.7 1108.78KJ.What will be heat of formation of carbondisulphide

The standard heat of formation of carbon disulphide (l) given that standard heat of combustion of - YouTube

The enthalpies of combustion of carbon and carbon monoxide are `-390 kJ mol^(-1)` and `-278 kJ mo - YouTube

On combustion carbon forms CO and CO2 The heat of formation of CO2 is - 393.5 kJ at of CO is - 110.5 kJ .The heat combustion of CO is

Enthalpy of combustion of carbon to `CO_(2)` is `-393.5 kJ mol^(-1)`. Calculate the heat release... - YouTube