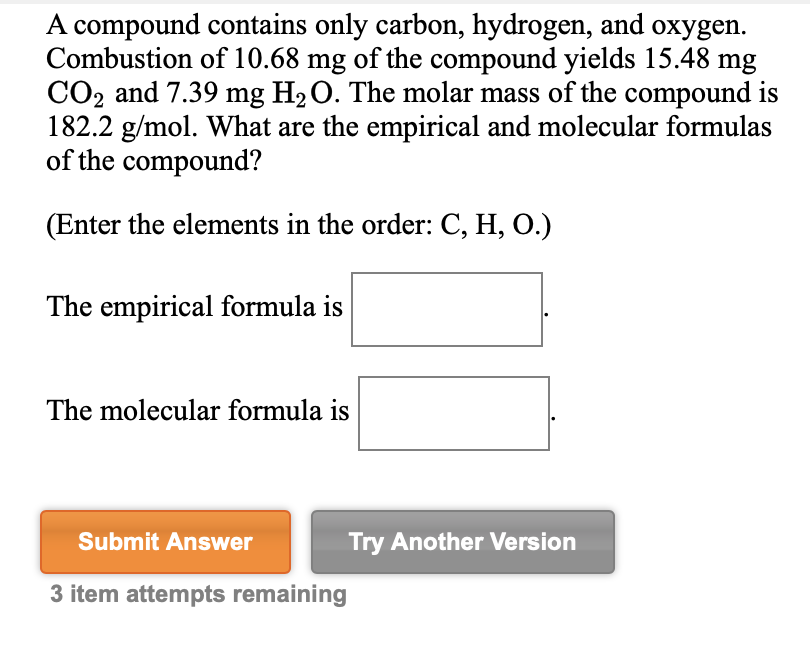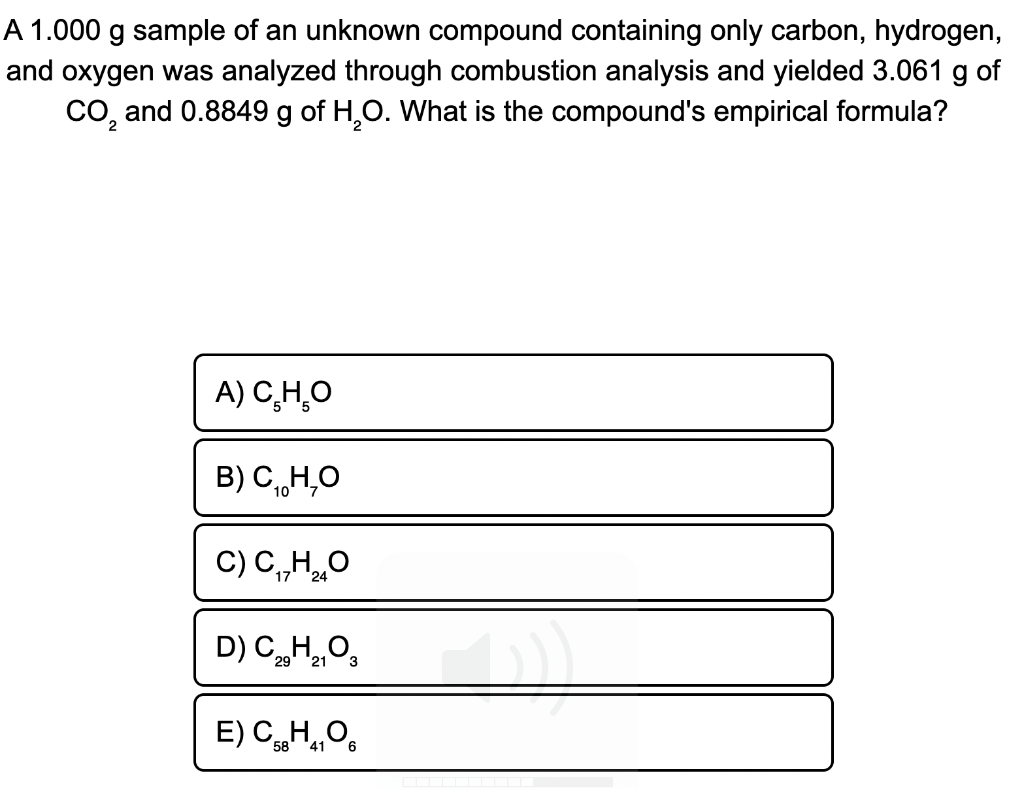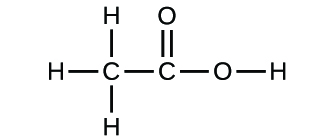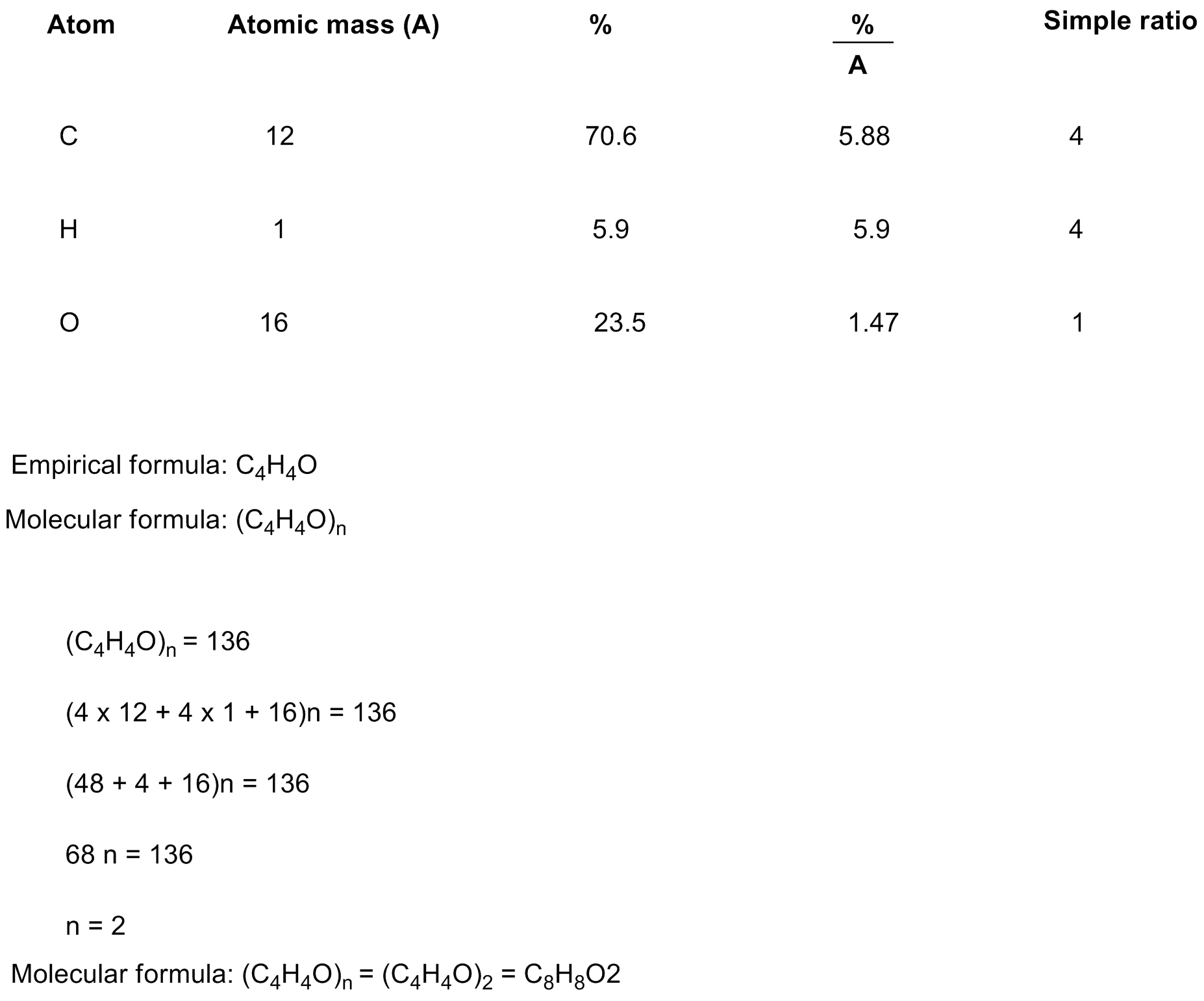
41) A compound that is composed of carbon, hydrogen, and oxygen contains 70.6% C, 5.9% H, and 23.5% O by mass. The molecular weight of the compound is 136 amu. What is

An organic compound contains carbon, hydrogen and oxygen. If the ratio percentage of C and - YouTube

SOLVED:A compound that contains only carbon, hydrogen, and oxygen is 48.64% C and 8.16% H by mass. What is the empirical formula of this substance?

SOLVED: A compound contains, by mass, 48.64% carbon, 8.16% hydrogen and 43.209 oxygen The empirical formula of this compound is: CzHsOz CaHgO3 CzH;O CzH;O

Carbon, hydrogen, oxygen, nitrogen, chlorine, and tin atoms are shown... | Download Scientific Diagram
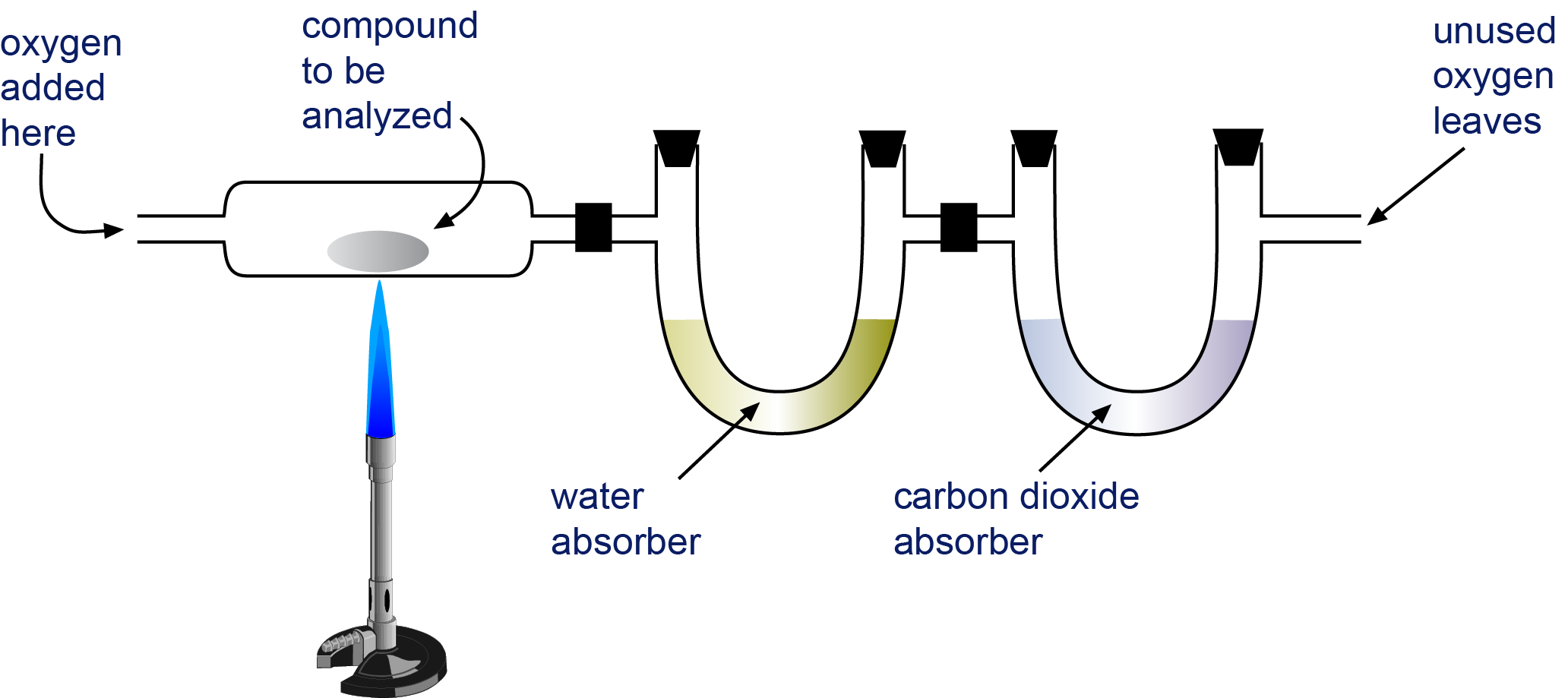
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are

A compound containing only carbon, hydrogen and oxygen was analyzed and found to contain 3.25 % hydrogen and 19.36 % carbon. What is the empricial formula of the compound? | Homework.Study.com

SOLVED: A compound that contains only carbon, hydrogen, and oxygen is 58.8% C and 9.87% by mass. What is the empirical formula of this substance?
An organic compound contains element C, H and oxygen. A 4.24 mg sample of compound is completely burnt in oxygen. It gives 8.45 mg of carbon dioxide and 3.46 mg of water.

An organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave`C,38.71% - YouTube