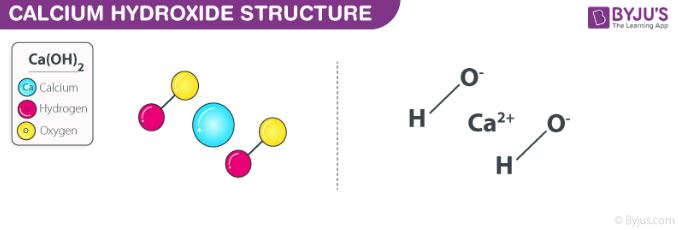
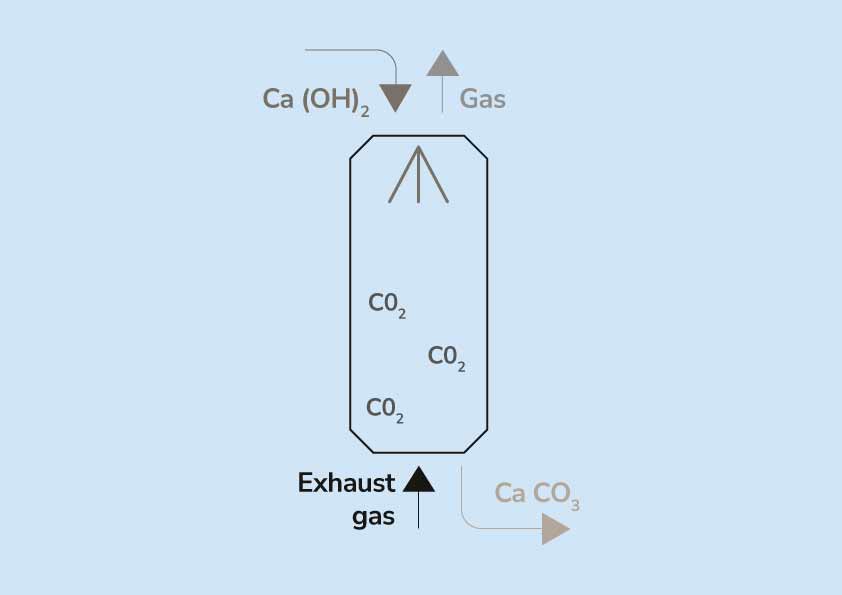
Write the balanced chemical equations for the following reactions.(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water(b) Zinc + Silver nitrate → Zinc nitrate + Silver(c) Aluminium + Copper chloride
The diagram below represents part of a set-up used to prepare and collect gas T. (a) Name two - Tutorke
Give a reason why calcium hydroxide solution is used to detect the presence of carbon (IV) oxide gas - Tutorke

why is a solution of potassium hydroxide used to absorb carbon dioxide evolved during the estimation of - Brainly.in

Reactions of carbon dioxide - Gas chemistry - (CCEA) - GCSE Chemistry (Single Science) Revision - CCEA - BBC Bitesize
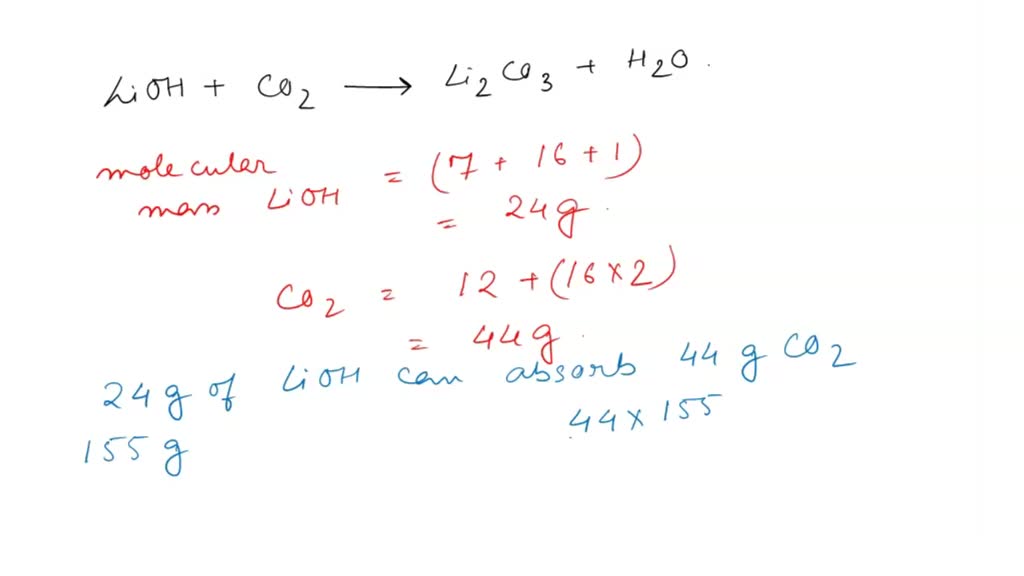
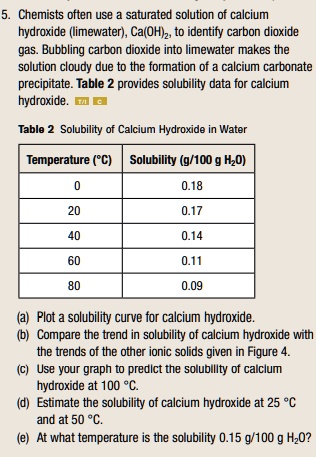
SOLVED: Alkali metal hydroxides are sometimes used to "scrub" excess carbon dioxide from the air in closed spaces (such as submarines and spacecraft). For example, lithium hydroxide reacts with carbon dioxide according

Lithium hydroxide is used in the space program to remove carbon dioxide (from respiration) in spacecraft. Specifically, lithium hydroxide, LiOH, reacts with carbon dioxide, CO_2, to form lithium carbo | Homework.Study.com

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride


.PNG)