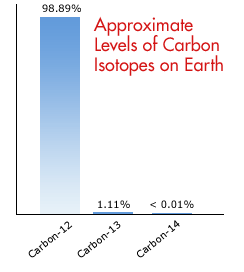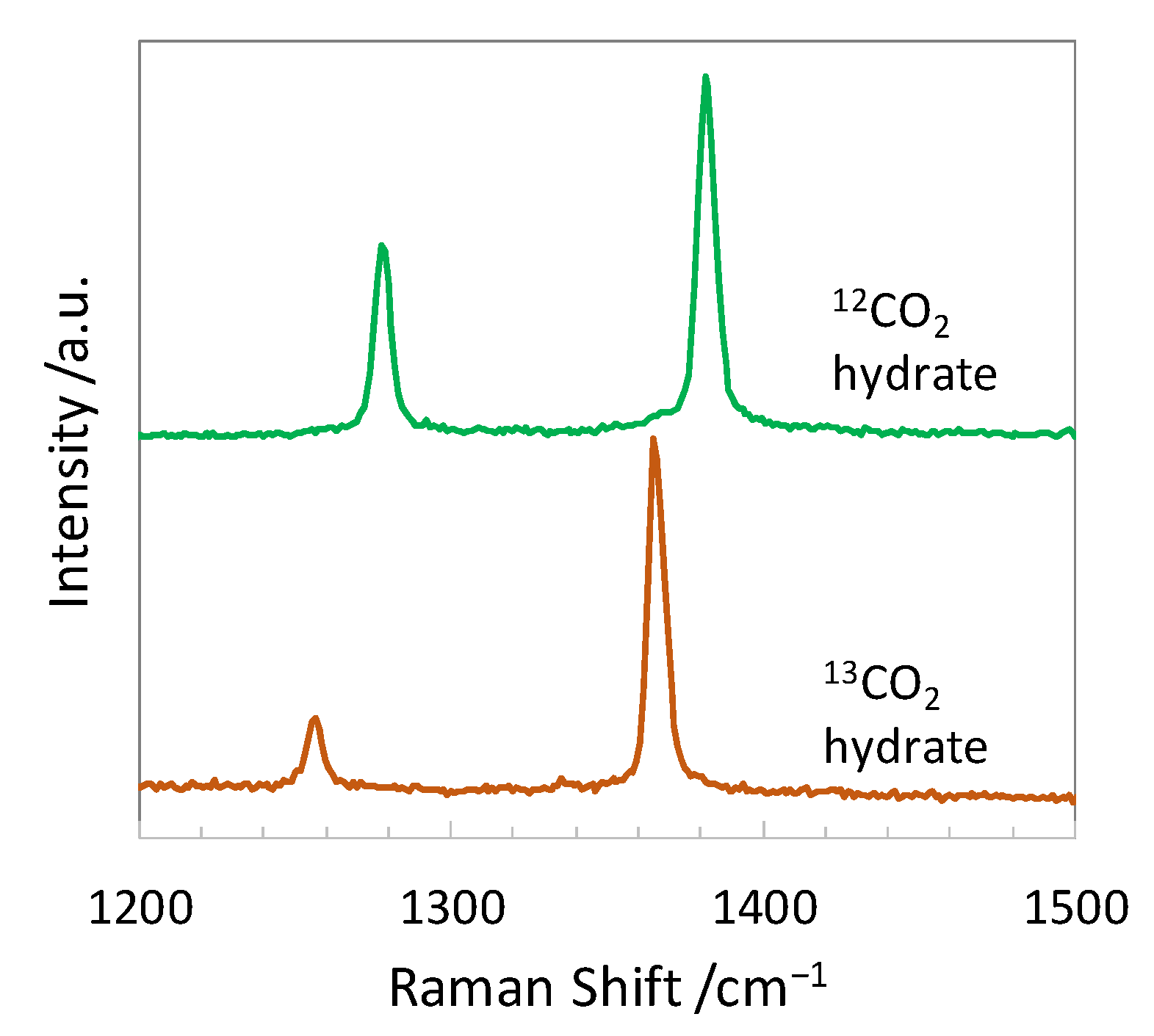
Molecules | Free Full-Text | Carbon Isotope Fractionation during the Formation of CO2 Hydrate and Equilibrium Pressures of 12CO2 and 13CO2 Hydrates
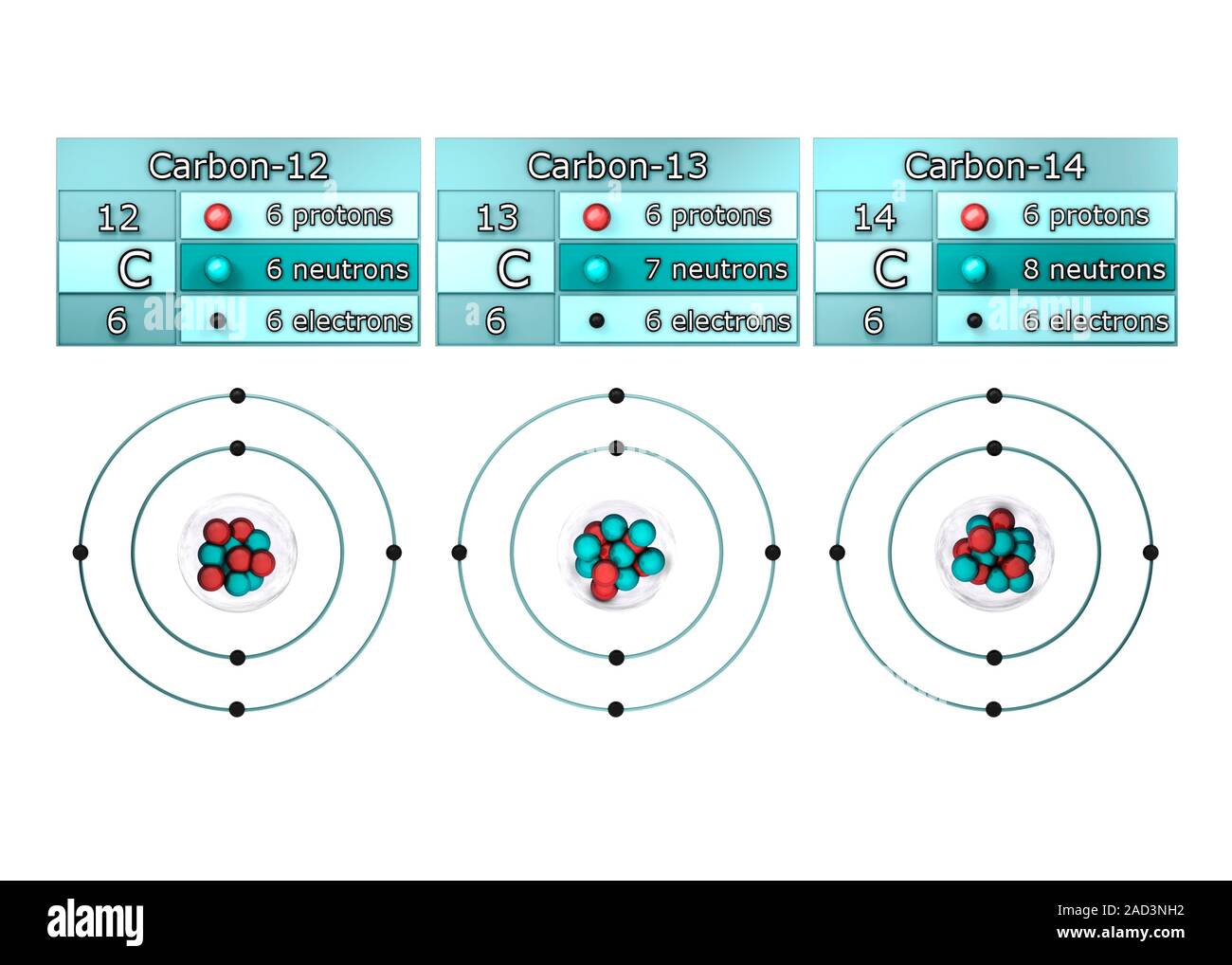
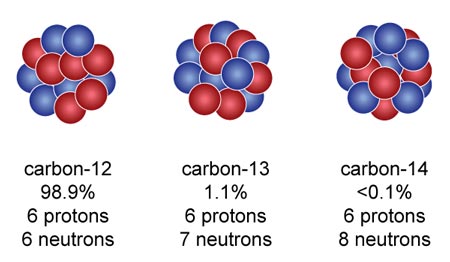
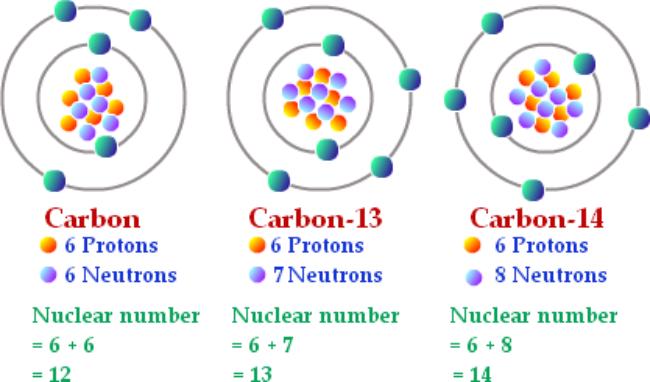
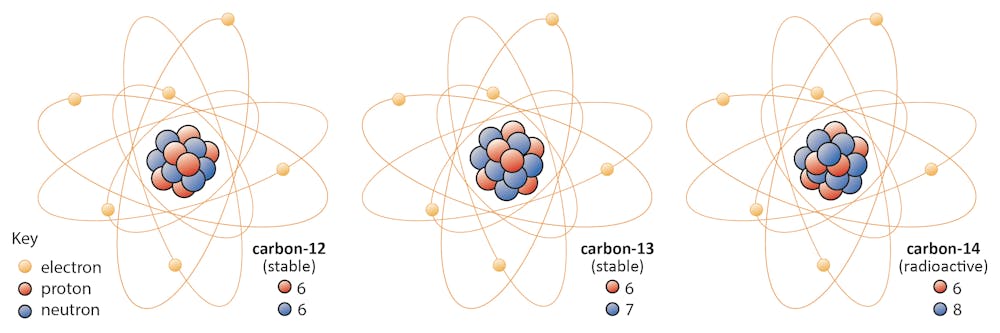
Isotopes of carbon. Illustration showing three isotopes of carbon: carbon-12, carbon-13 and carbon-14. Isotopes are forms of an element that contain d Stock Photo - Alamy

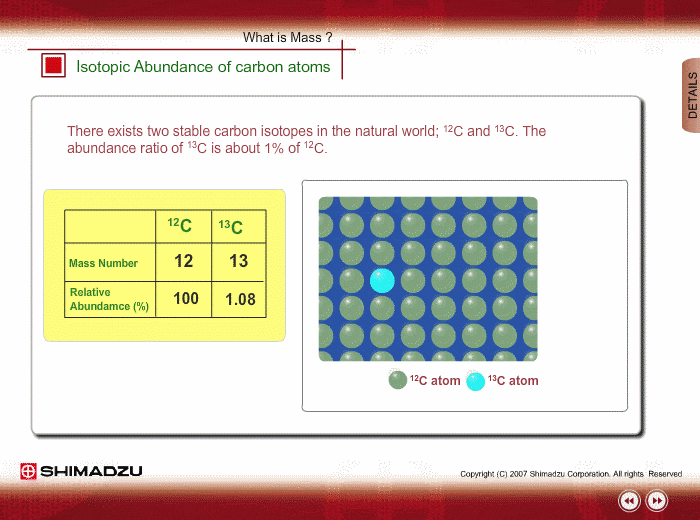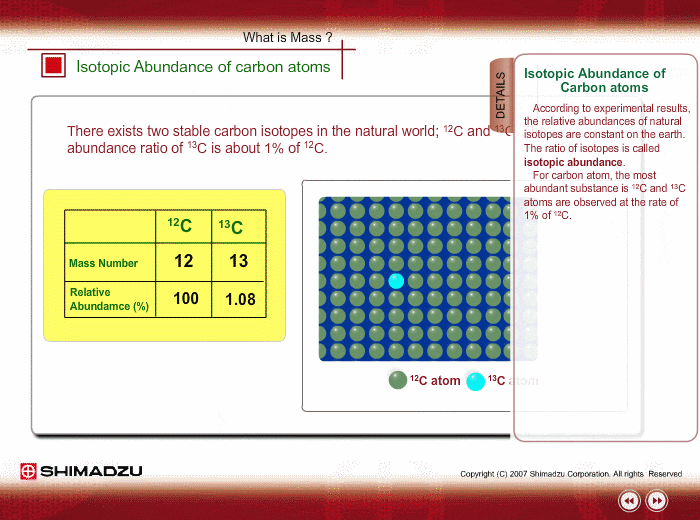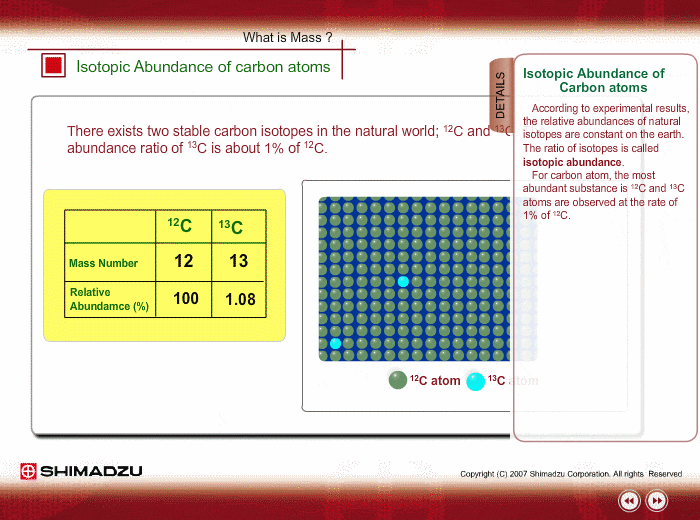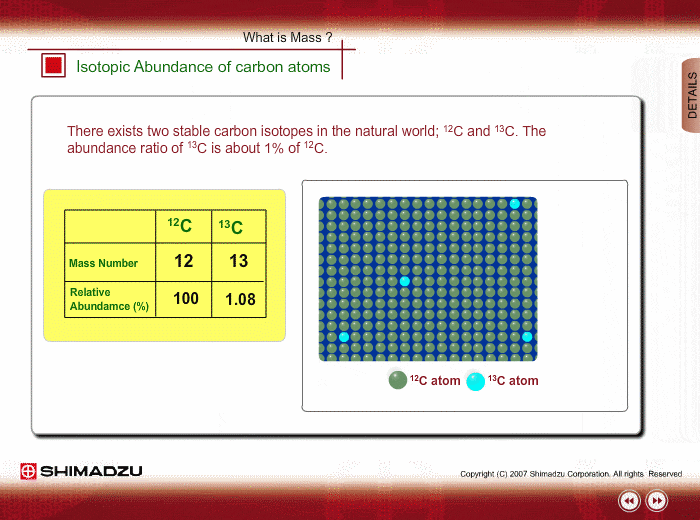
Chlorine has two isotopes of atomic mass units 34.97 and 36.97. The relative abundance of an isotope is 0.755 and 0.245 respectively. Find the average atomic mass of chlorine.

Changes to Carbon Isotopes in Atmospheric CO2 Over the Industrial Era and Into the Future - Graven - 2020 - Global Biogeochemical Cycles - Wiley Online Library