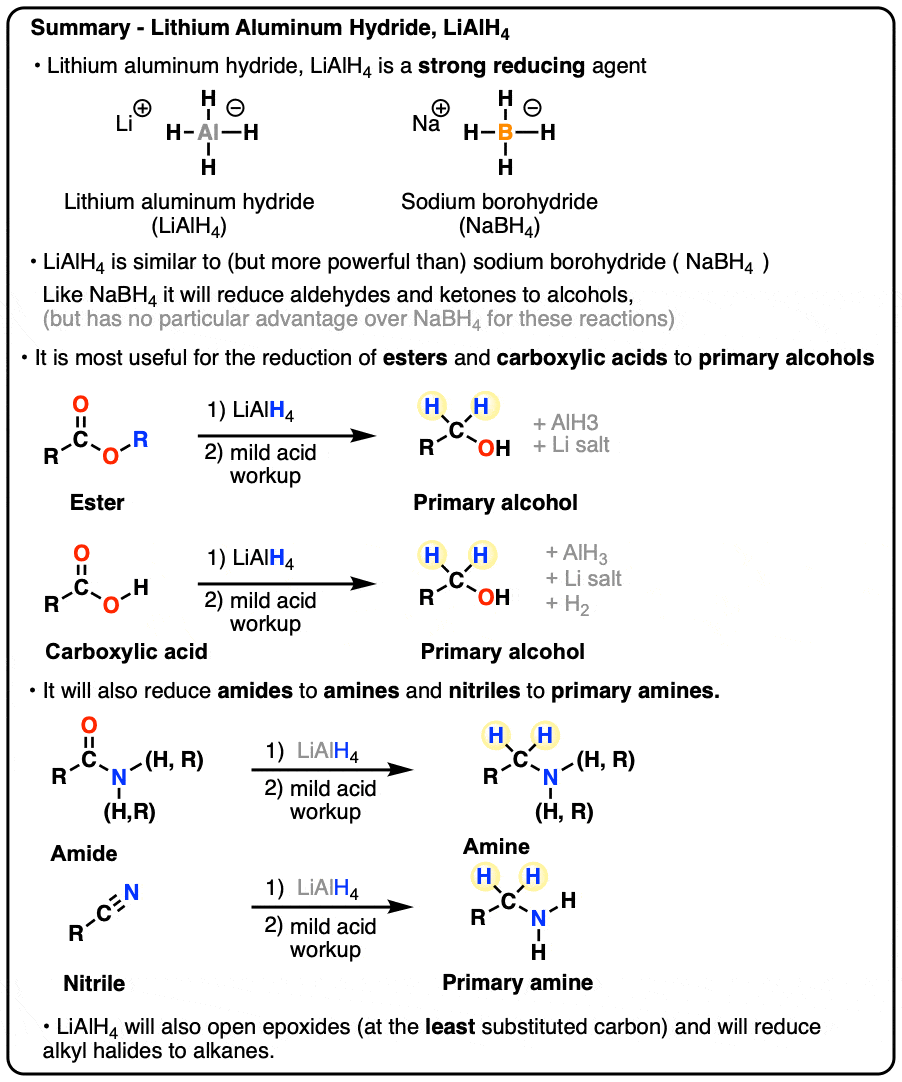
Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives – Master Organic Chemistry

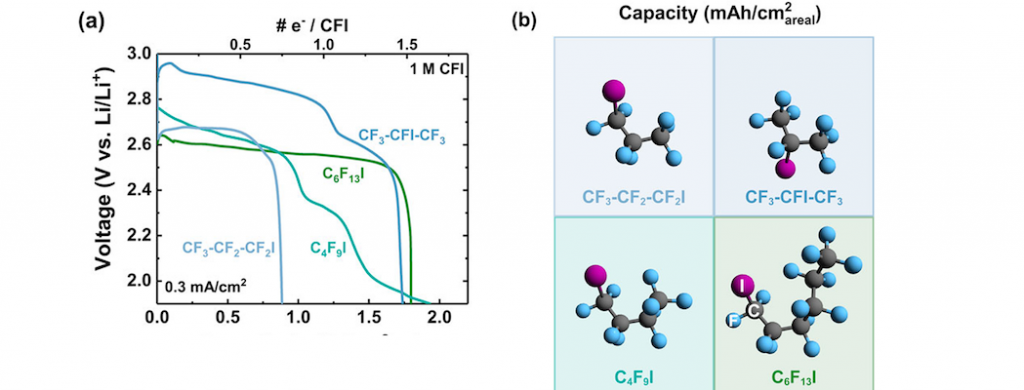
High sulfur-containing carbon polysulfide polymer as a novel cathode material for lithium-sulfur battery | Scientific Reports

Carbon-sulfur double bond electrolyte additives as redox mediator for lithium-oxygen batteries - ScienceDirect

Lithium Enolates in the Enantioselective Construction of Tetrasubstituted Carbon Centers with Chiral Lithium Amides as Noncovalent Stereodirecting Auxiliaries | Journal of the American Chemical Society
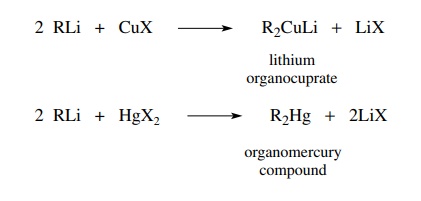
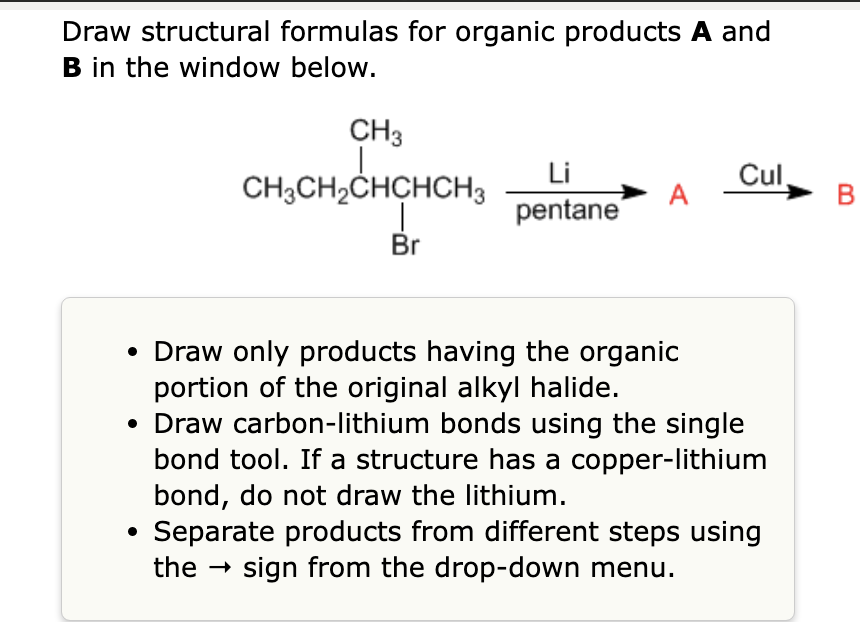
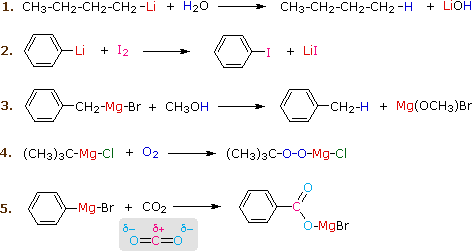
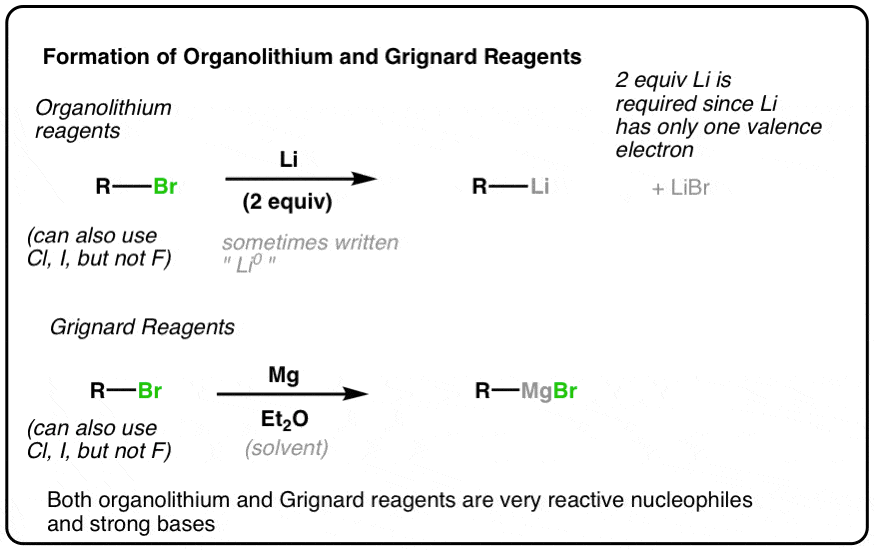
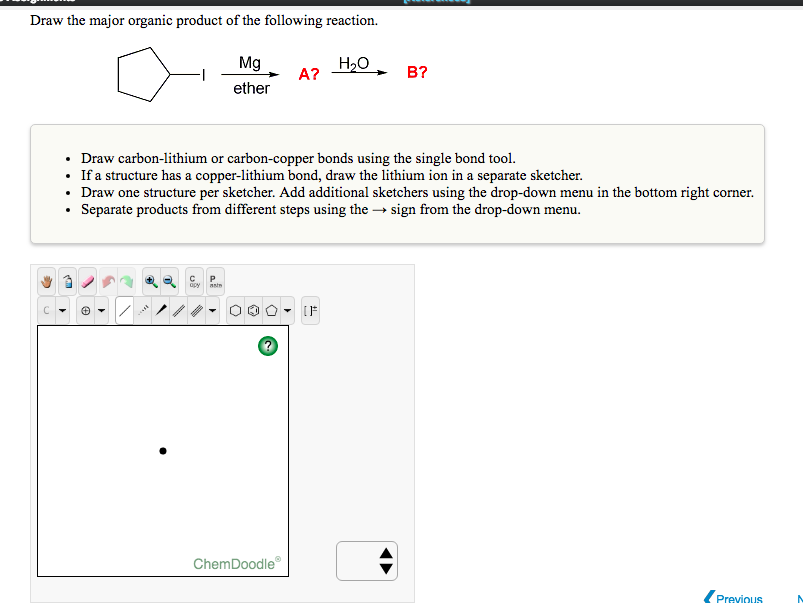
Generation of Nucleophilic Carbon Reagents - Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles | Organic Chemistry

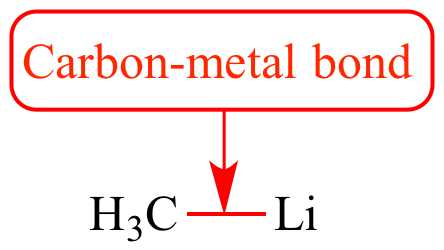
Lithium Bonds in Lithium Batteries - Chen - 2020 - Angewandte Chemie International Edition - Wiley Online Library