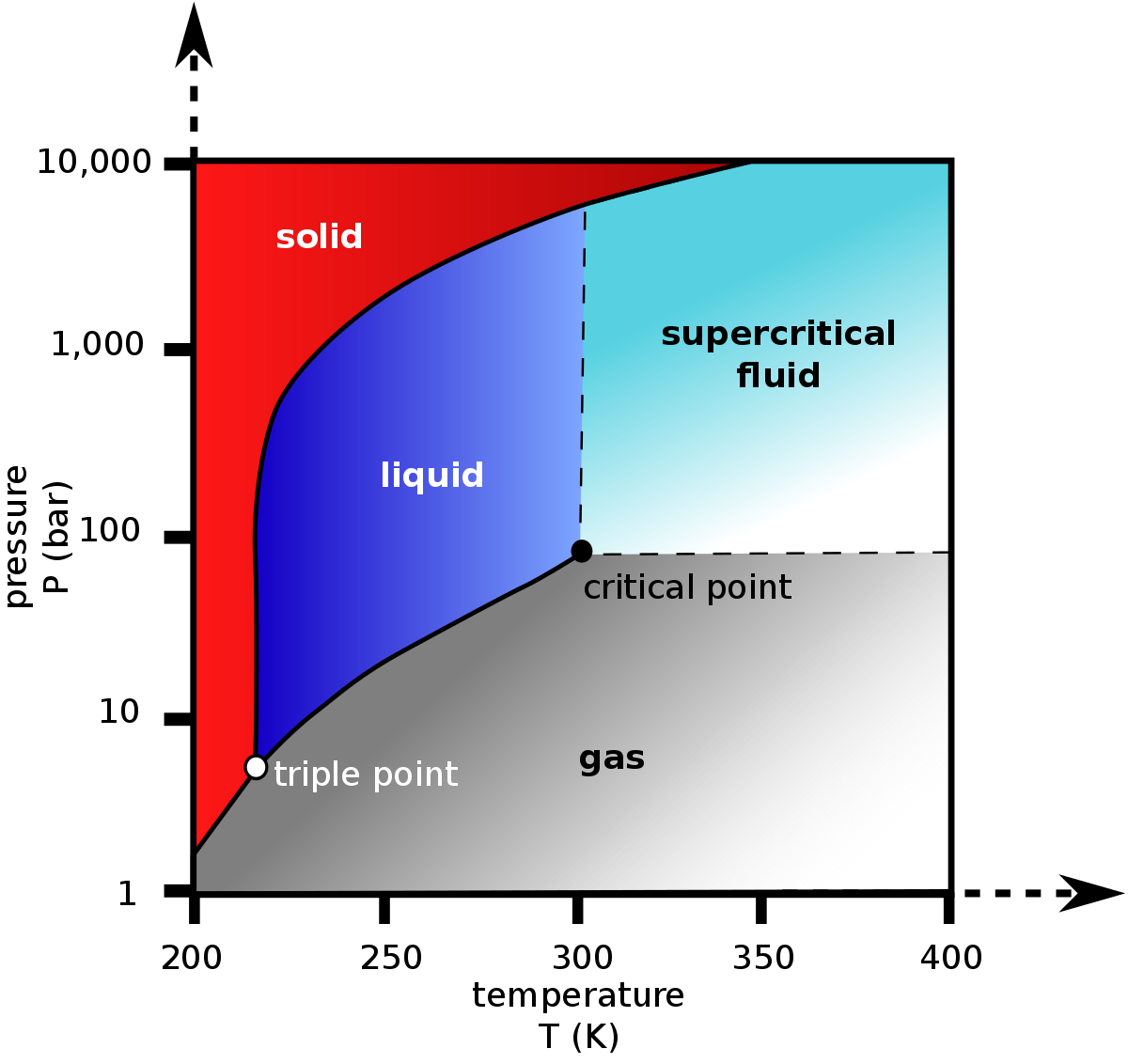
SOLVED: Carbon Dioxide Water Freezing Point (K) Boiling Point Sublimation Point N/A N/A 273 373 195 N/A Liquid Specific Heat Capacity (J/kg-K) Solid Specific Heat Capacity (J/kg K) Latent Heat of Fusion (

The heat capacity of nickel carbonyl and the thermodynamics of its formation from nickel and carbon monoxide - Journal of the Chemical Society (Resumed) (RSC Publishing)

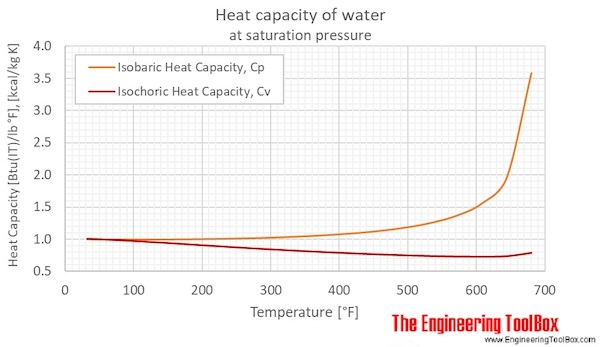
Isobaric heat capacity (Cp) measurements of supercritical fluids using flow calorimetry: equipment design and experimental validation with carbon dioxide, methanol, and carbon dioxide-methanol mixtures - ScienceDirect

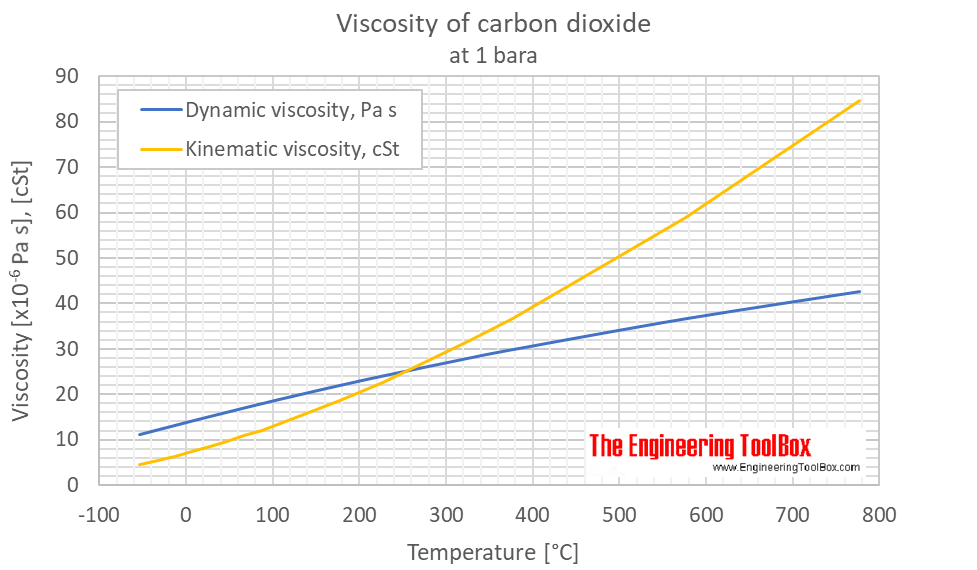
Carbon dioxide heat capacity vs. temperature for different pressures:... | Download Scientific Diagram
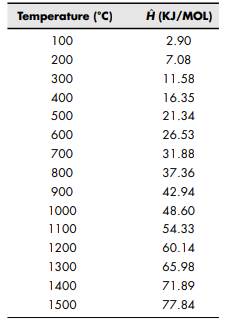
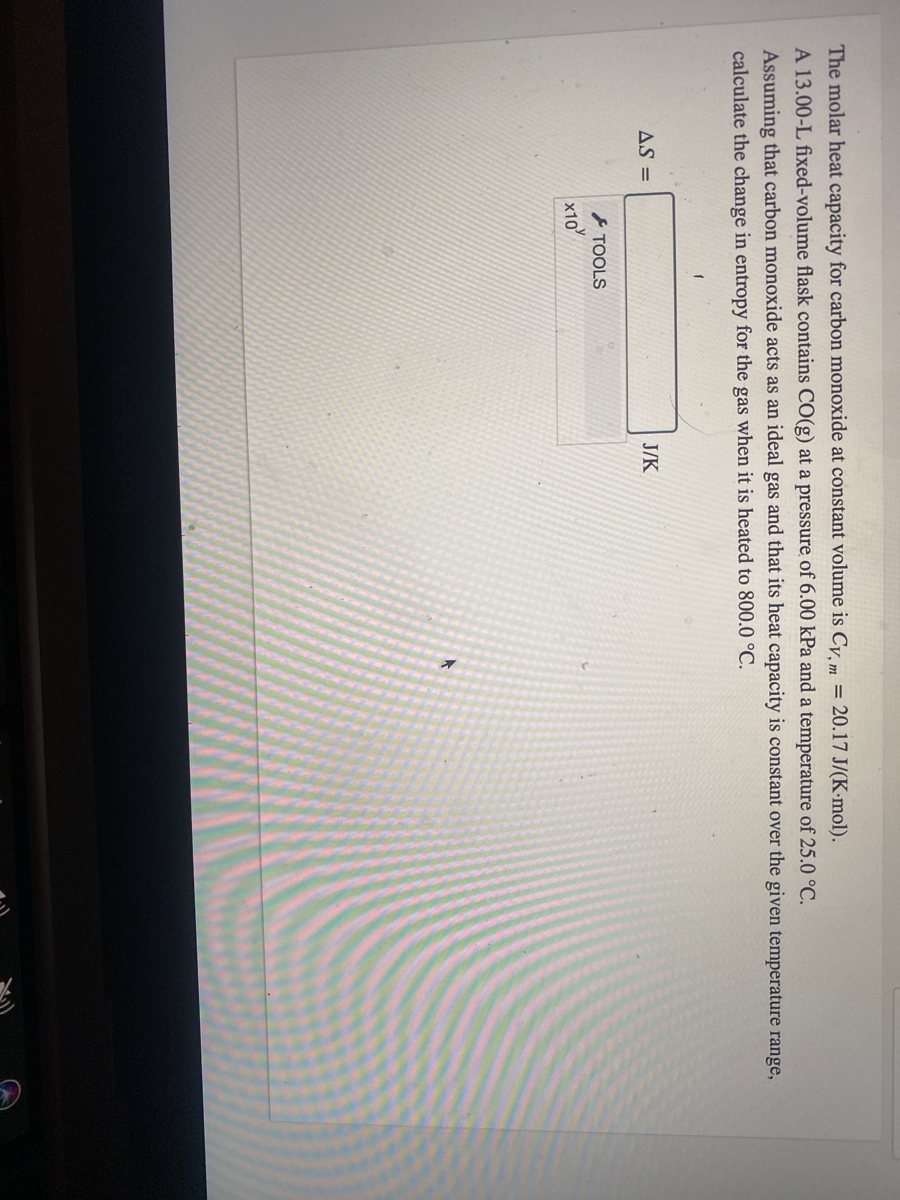
Solved) - Calculating Heat Capacity I The heat capacity at constant pressure... - (1 Answer) | Transtutors
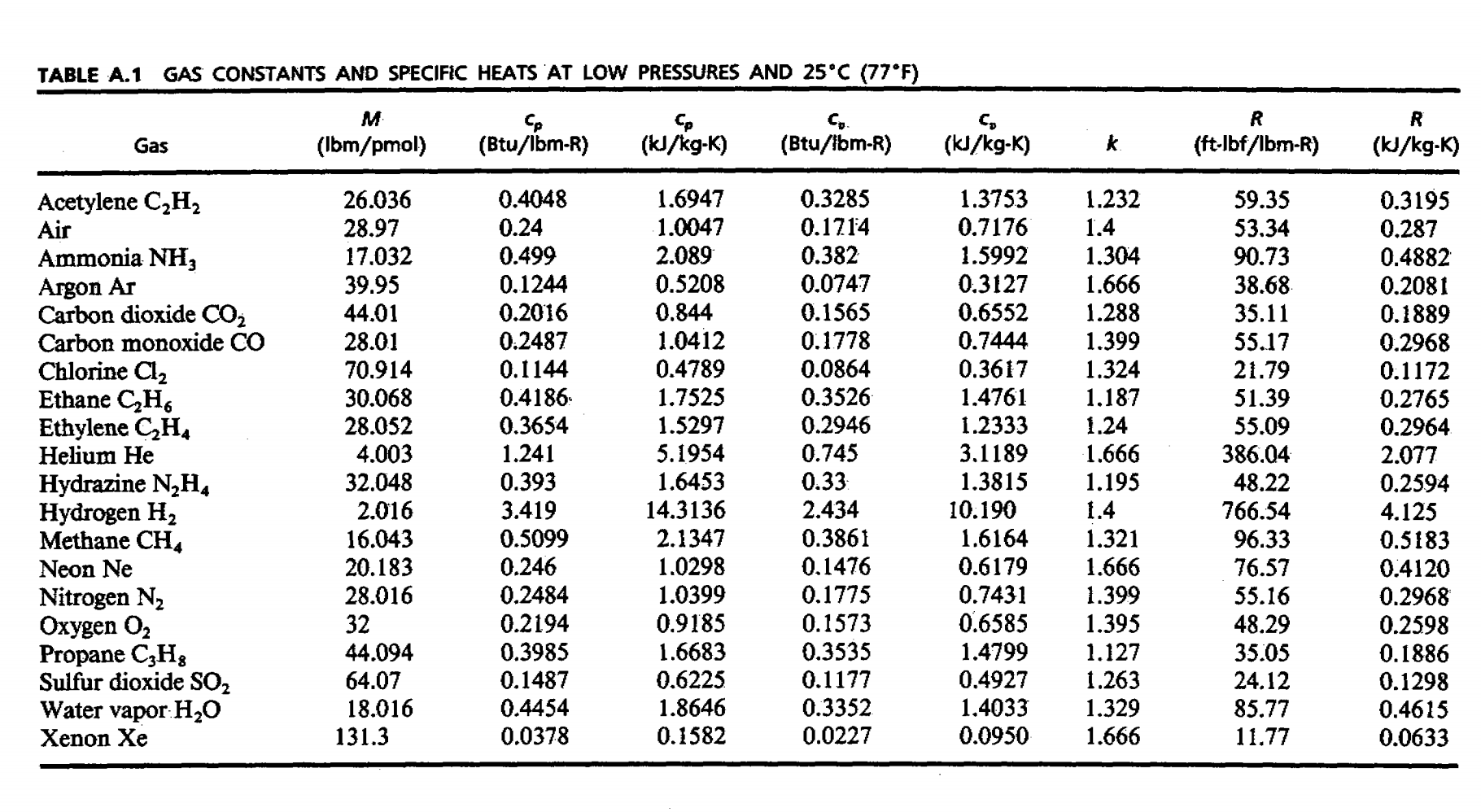
![PDF] Uncertainty estimation of the mean specific heat capacity for the major gases contained in biogas | Semantic Scholar PDF] Uncertainty estimation of the mean specific heat capacity for the major gases contained in biogas | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/15f896b0b9a5fbe30c19255b491bc89b7dbeca8a/6-Table4-1.png)
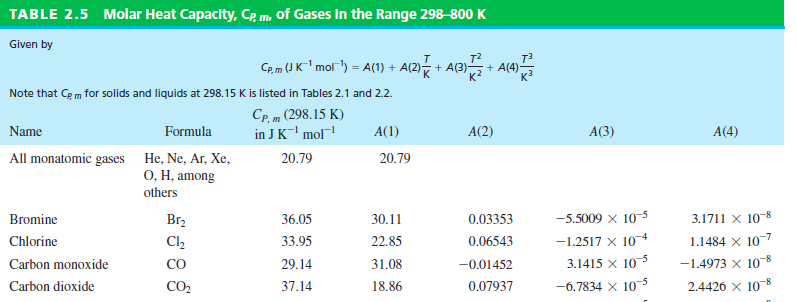
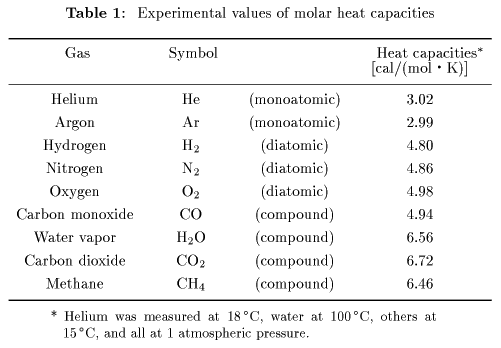
PDF] Uncertainty estimation of the mean specific heat capacity for the major gases contained in biogas | Semantic Scholar

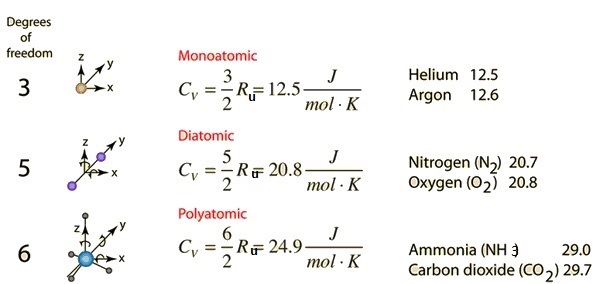
quantum mechanics - What is the high temperature molar heat capacity of $\mathrm{CO}_2$ (carbon dioxide)? - Physics Stack Exchange
Heat of combustion of carbon monoxide is 283.5 kJ/mole the heat released when 55g of carbon dioxide formed from carbon monoxide
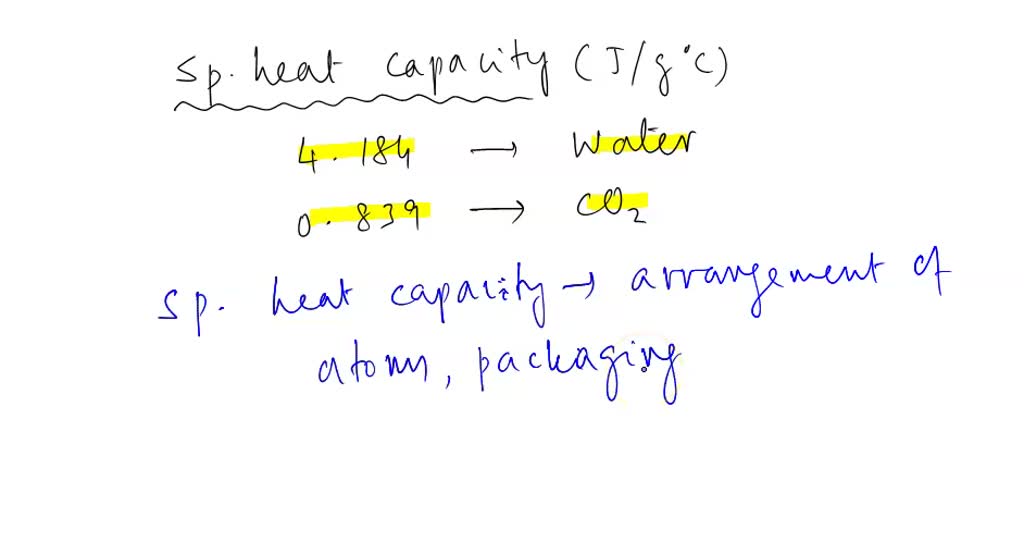
SOLVED: Consider the specific heat capacities of carbon dioxide and water below: Substance Specific Heat Capacity (J/g·K) Water 4.184 CO2 0.839 Based on the chemical structure and properties of water vs. carbon