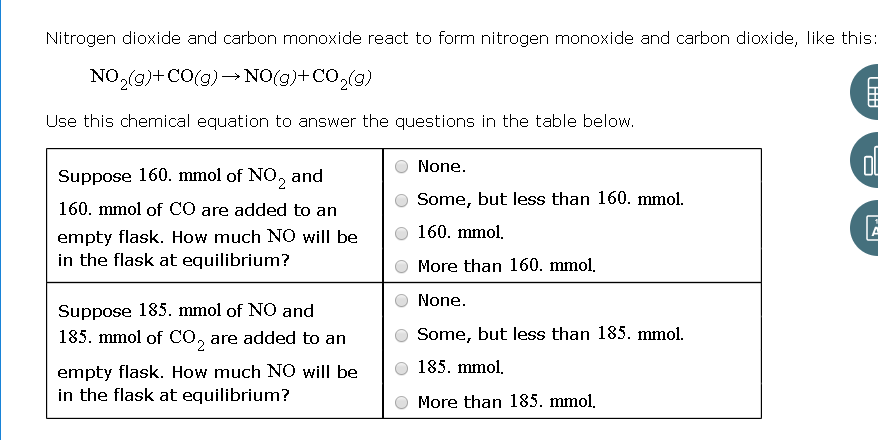
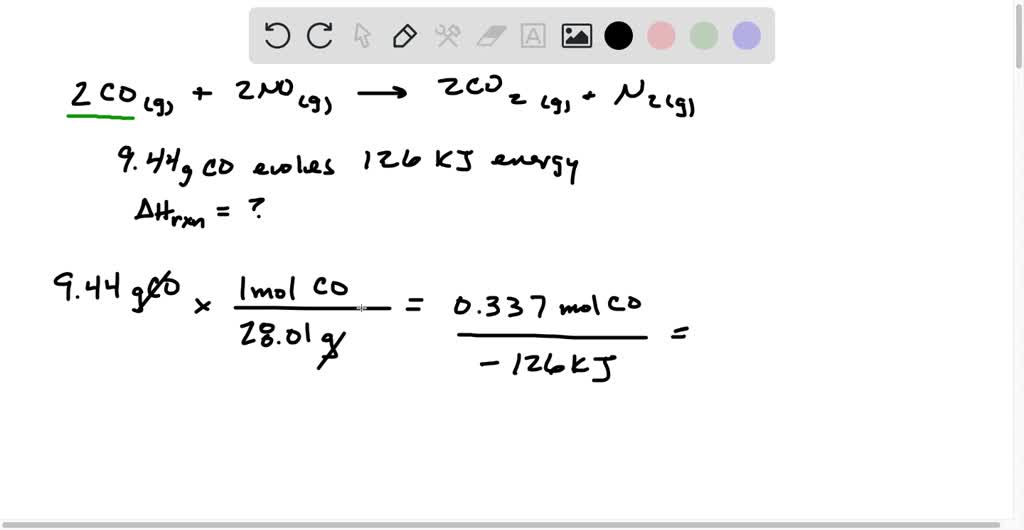Amount of carbon monoxide (CO) and nitrogen oxides (NO x ) in smoke... | Download Scientific Diagram
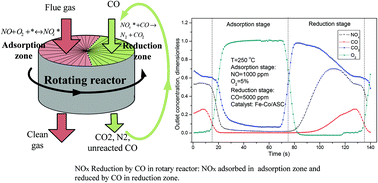
Nitrogen oxides reduction by carbon monoxide over semi-coke supported catalysts in a simulated rotary reactor: reaction performance under dry conditions - Green Chemistry (RSC Publishing)
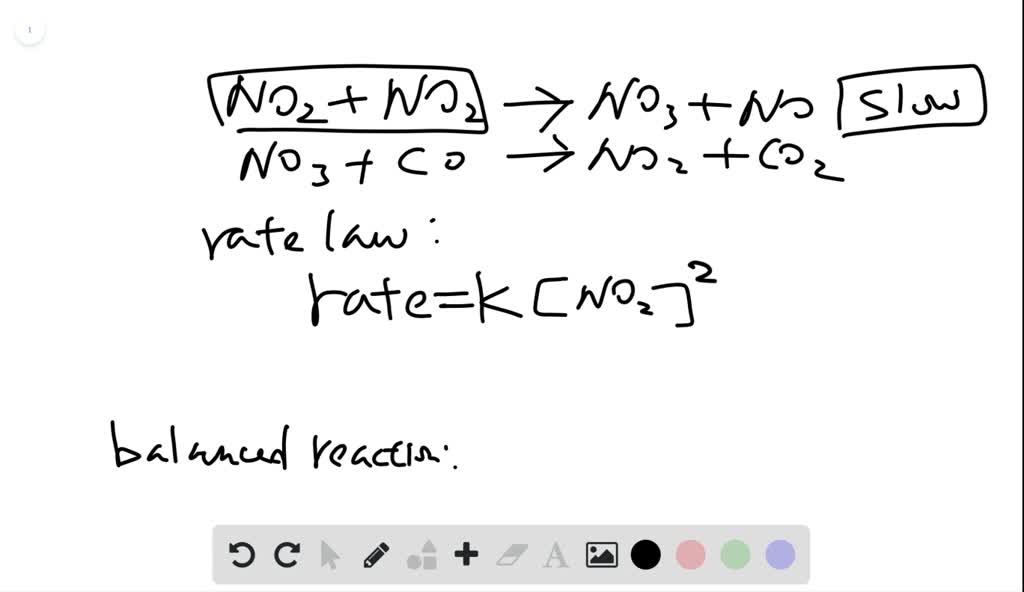
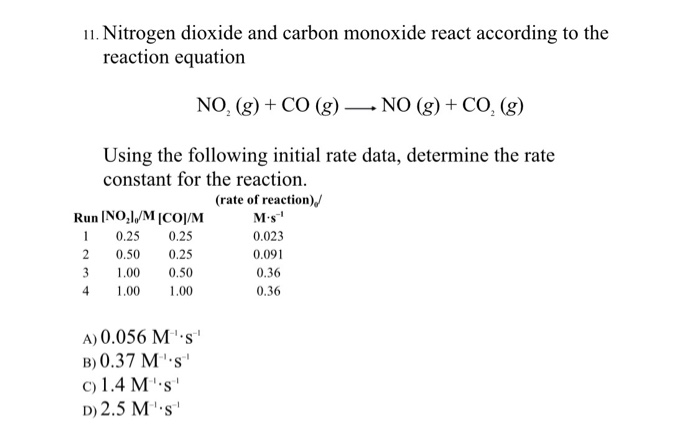
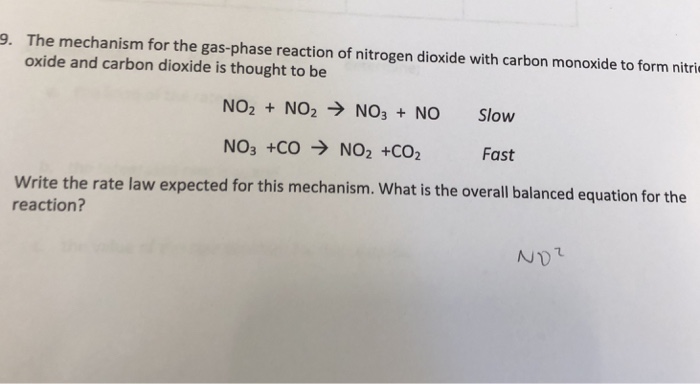
SOLVED: The mechanism for the gas-phase reaction of nitrogen dioxide with carbon monoxide to form nitric oxide and carbon dioxide is thought to be NO2+NO2⟶NO3+NO NO3+CO⟶NO2+CO2 Write the rate law expected for
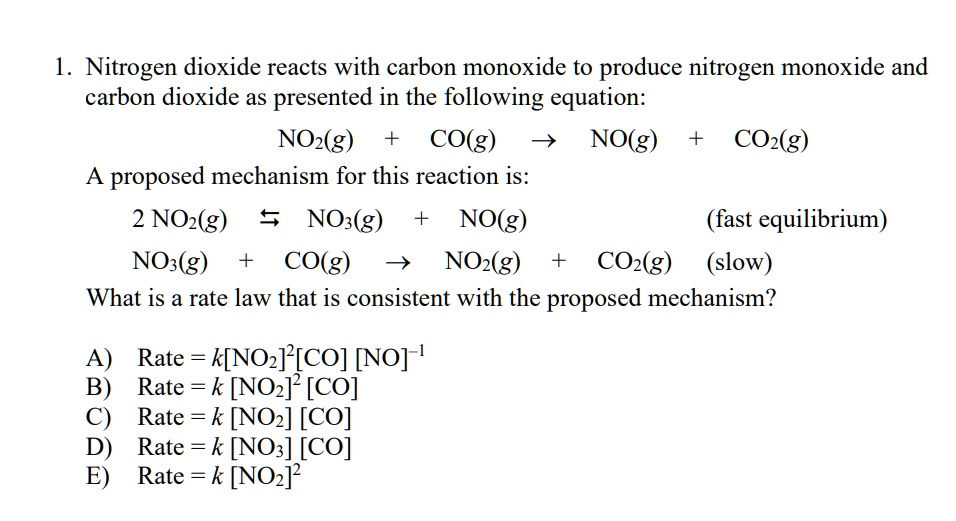
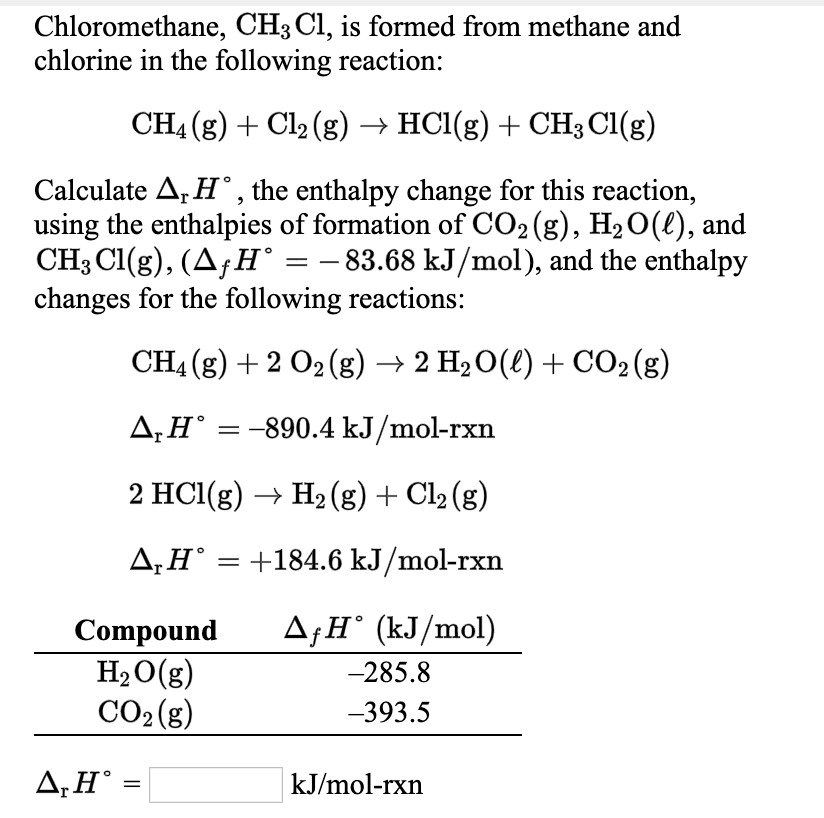
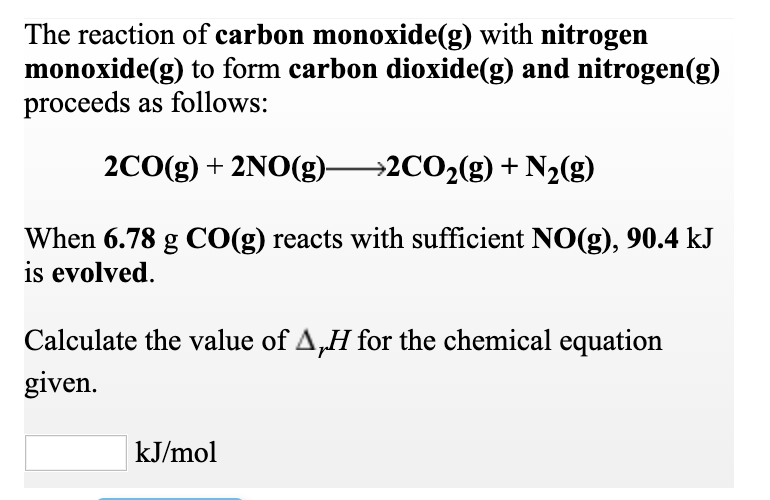
SOLVED: Nitrogen dioxide reacts with carbon monoxide to produce nitrogen monoxide and carbon dioxide as presented in the following equation: NOz(g) cO(g) NO(g) COz(g) A proposed mechanism for this reaction is: 2

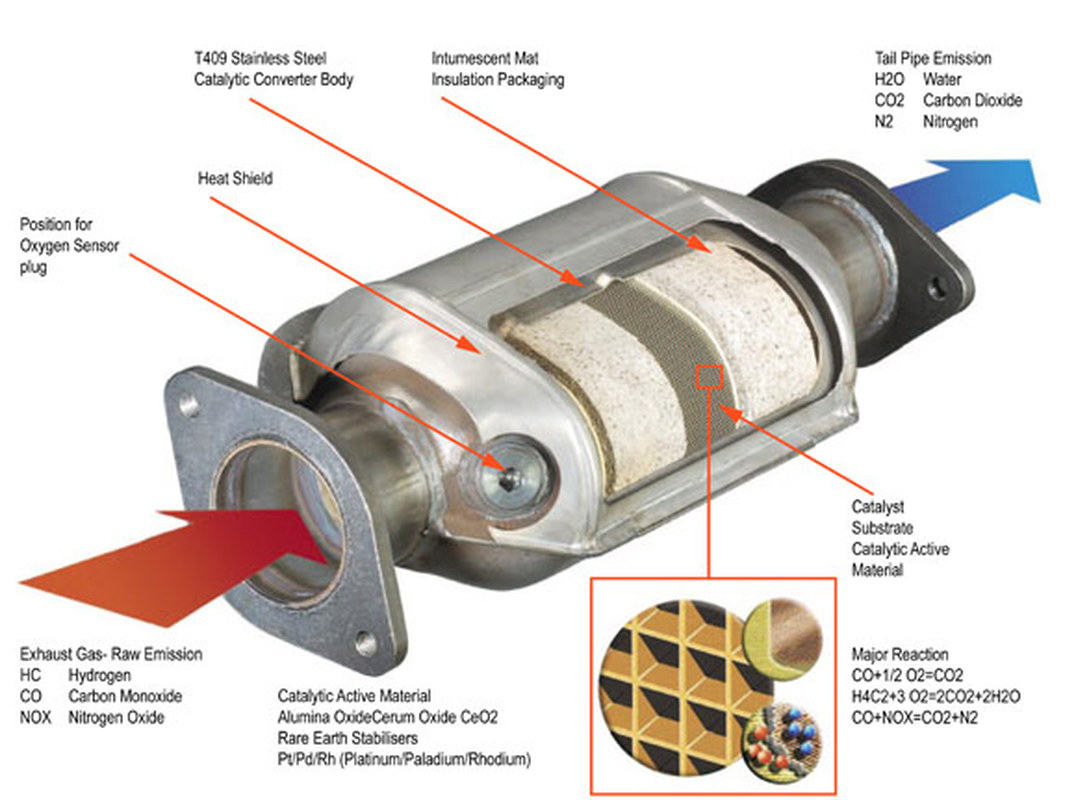
A review of selective catalytic reduction of nitrogen oxides with hydrogen and carbon monoxide - ScienceDirect