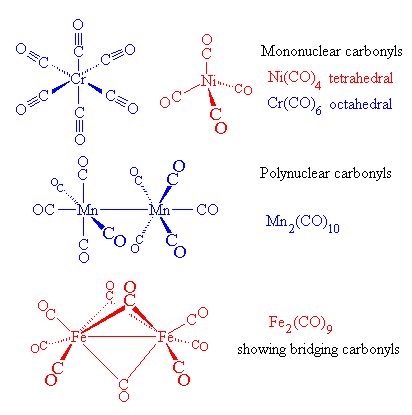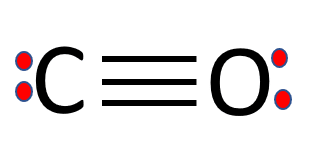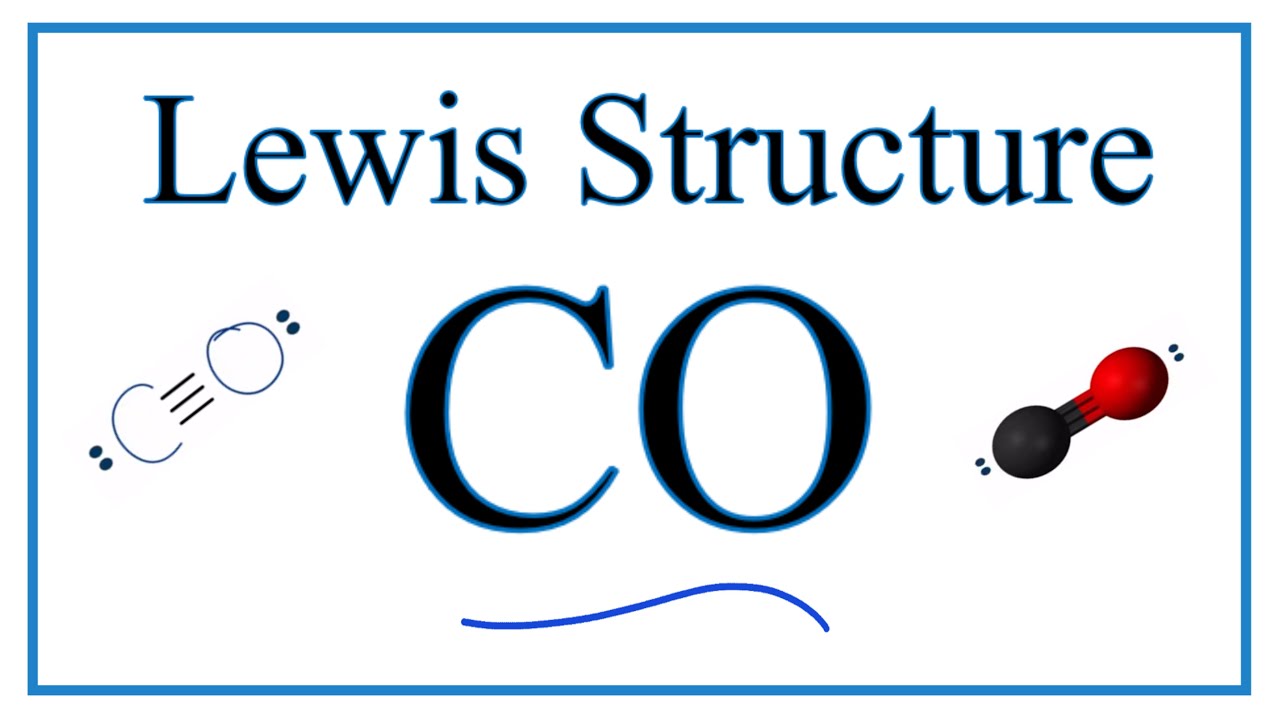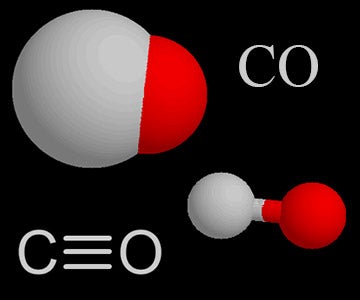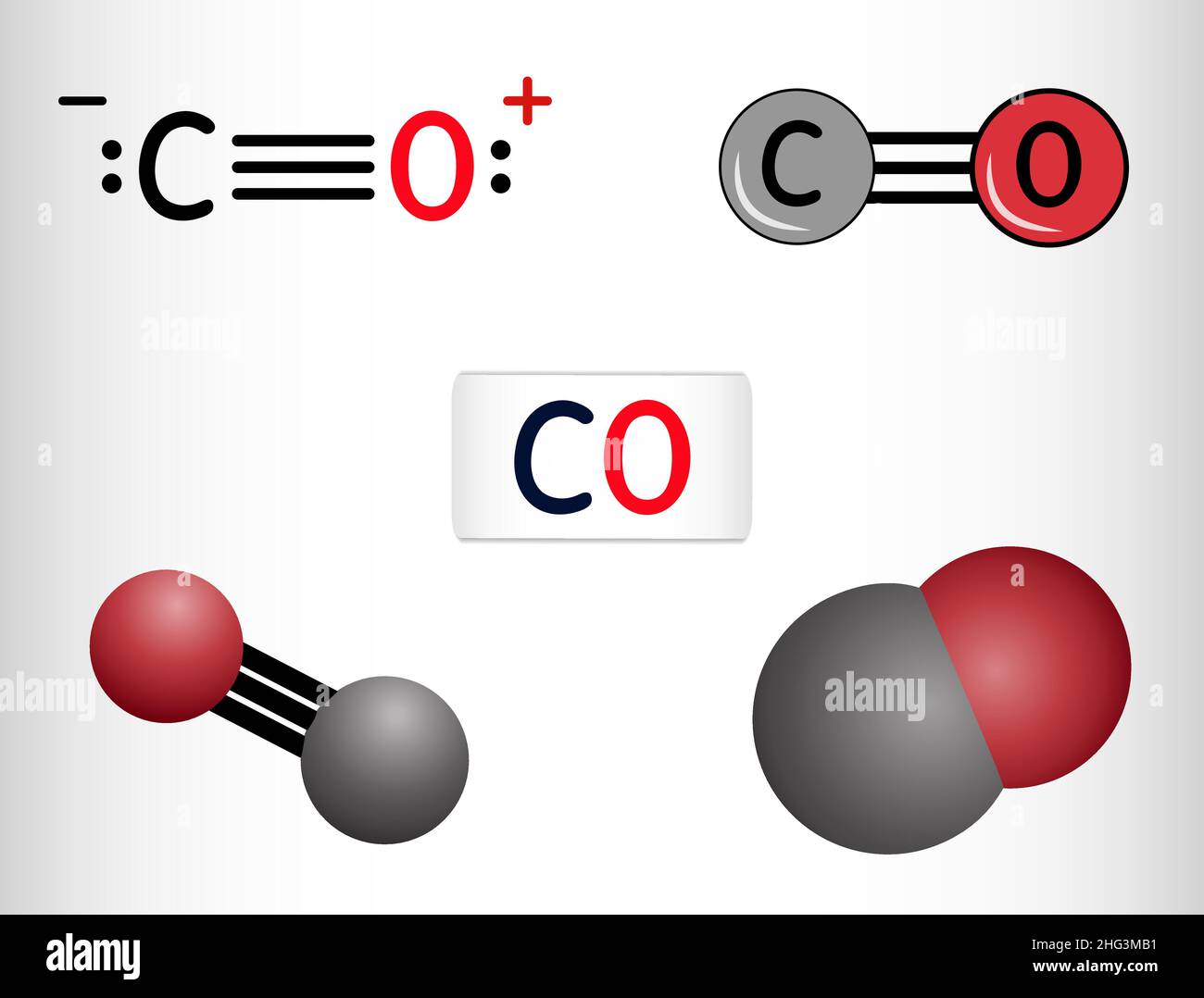
Carbon monoxide, CO molecule. Сarbon and oxygen atoms are connected by a triple bond. Structural chemical formula and molecule model. Vector illustrat Stock Vector Image & Art - Alamy

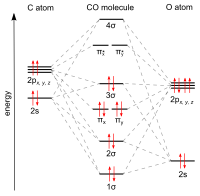
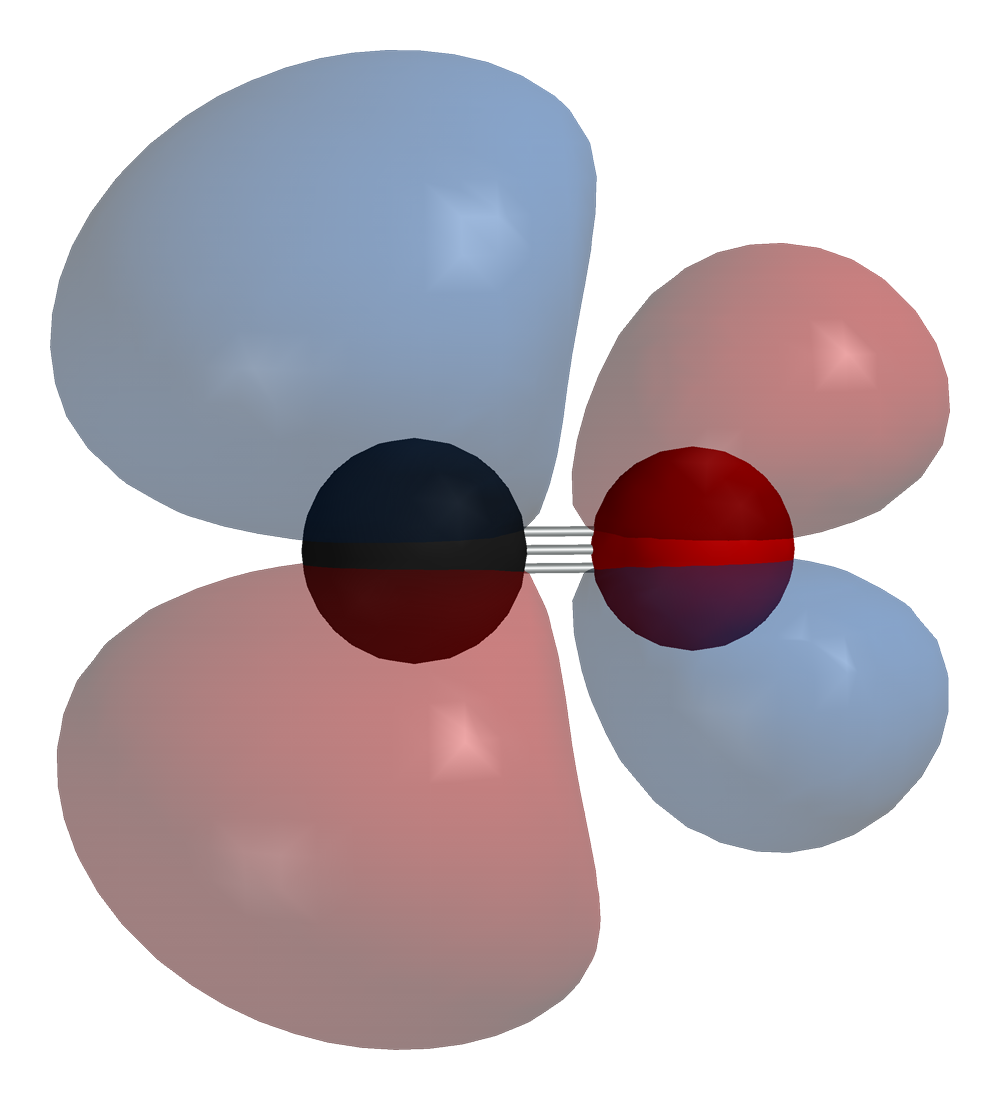
Why does carbon in carbon monoxide forms triple bonds with oxygen whereas oxygen never forms never forms triple bonds except in coordinate bonds? - Quora

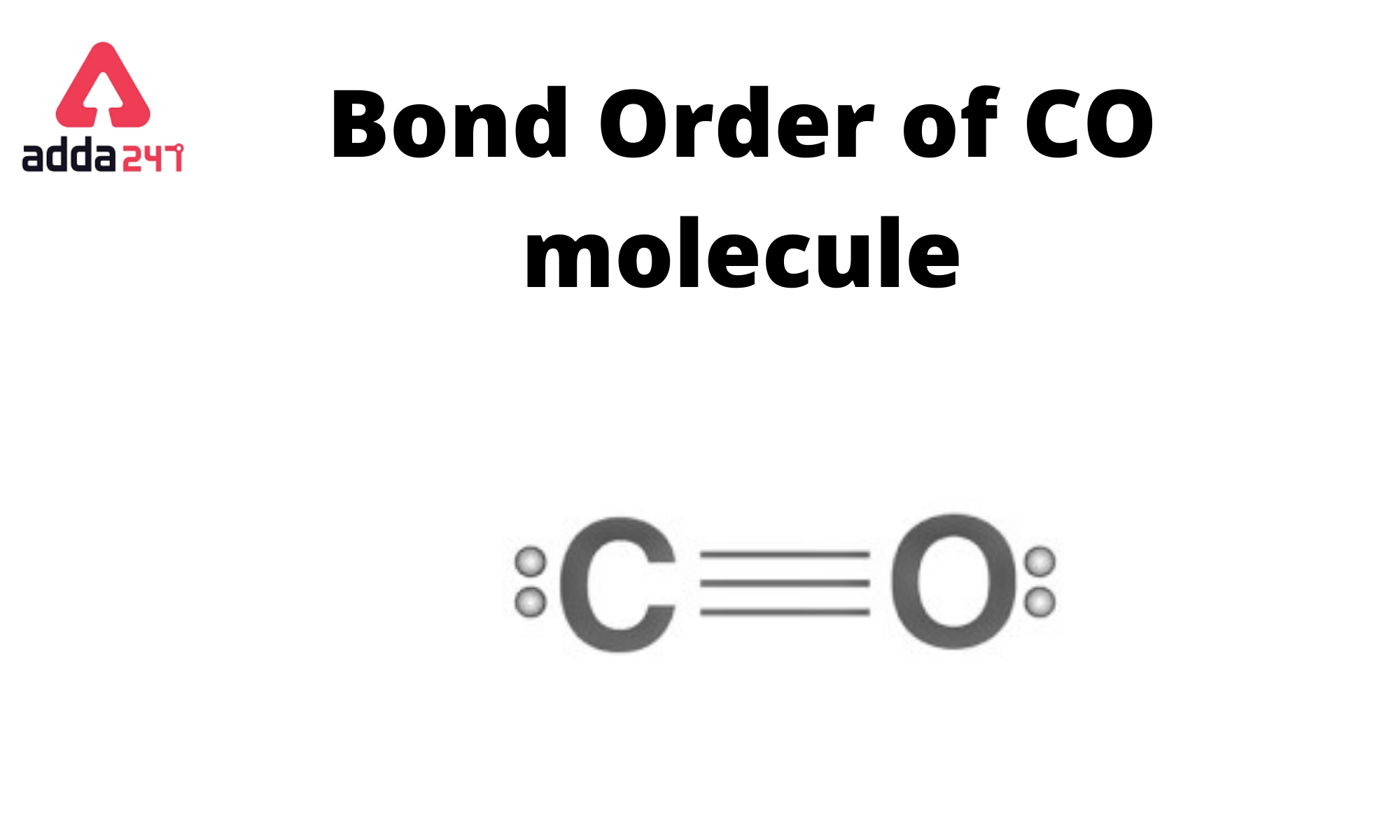
Carbon Monoxide, CO Molecule. Сarbon and Oxygen Atoms are Connected by a Triple Bond that Consists of Two Pi Bonds and One Sigma Stock Vector - Illustration of icon, molecular: 239578219
How can carbon monoxide be a stable molecule when it forms only three bonds with it's only other atom, oxygen? - Quora