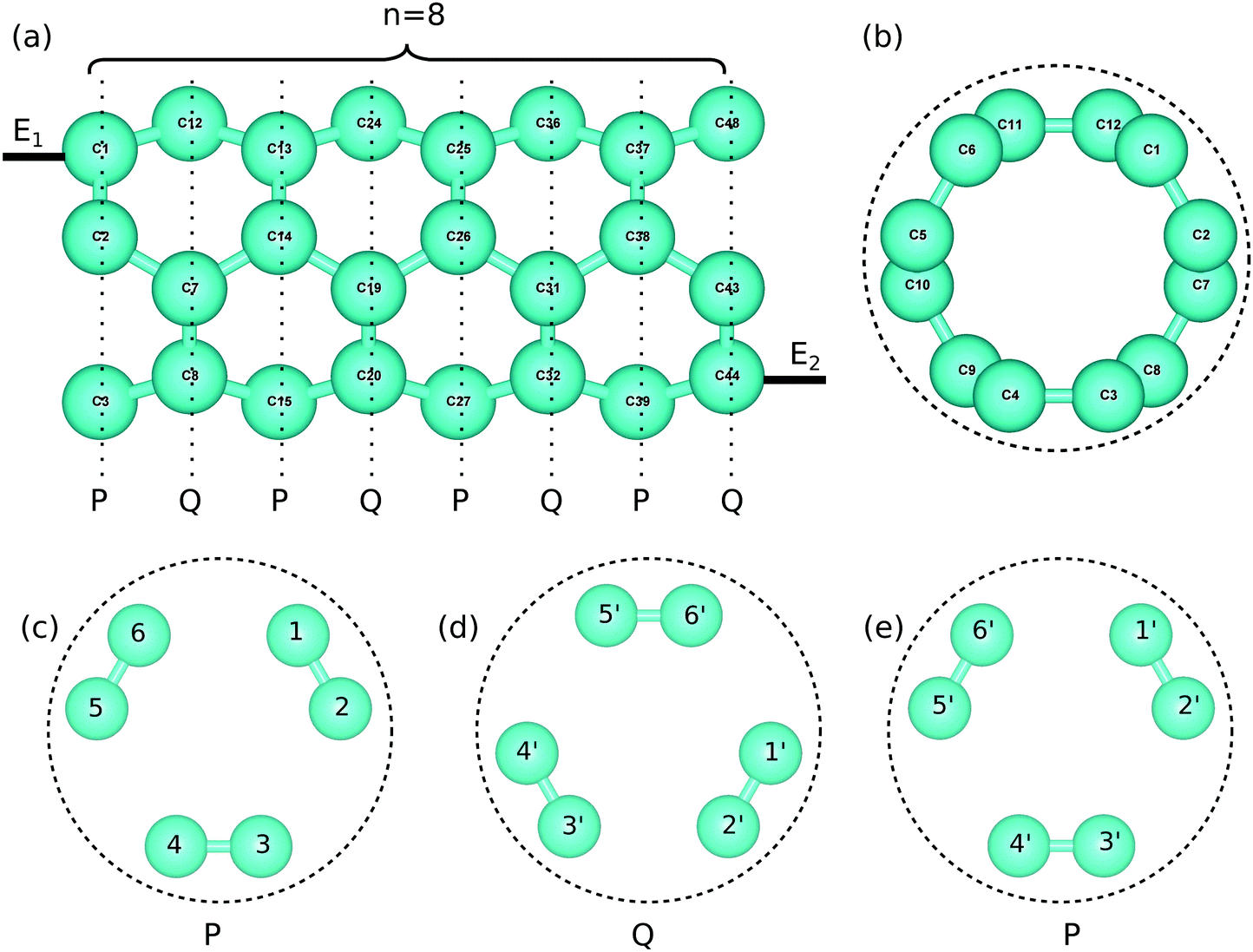
Graphene nanocomposites and applications in electrochemical energy storage materials - ScienceDirect

4. Fullerenes, bucky balls, carbon nanotubes molecular structure formula uses applications gcse igcse gce A Level AS A2 chemistry revision notes
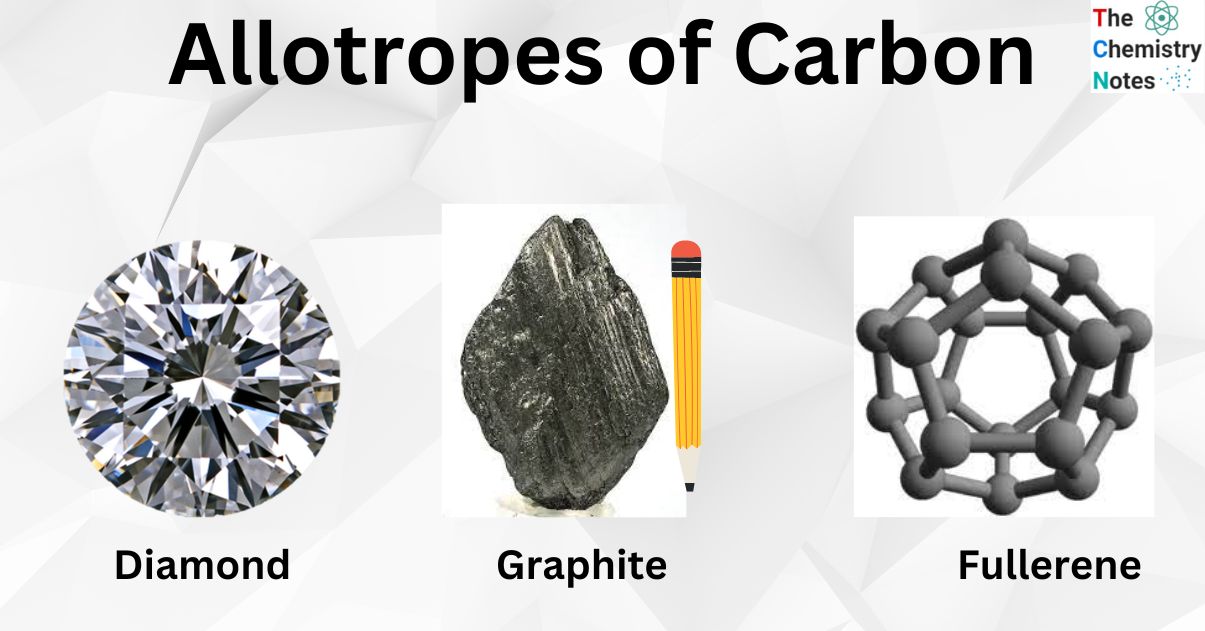
Both diamond and graphite are made from carbon. However, diamond is considered the hardest material, while graphite is brittle and slippery. What is this difference, from an atomic bonding view point? -
![SuperSimple Chemistry: The Ultimate Bitesize Study Guide [1 ed.] 1465493239, 9781465493231 - DOKUMEN.PUB SuperSimple Chemistry: The Ultimate Bitesize Study Guide [1 ed.] 1465493239, 9781465493231 - DOKUMEN.PUB](https://dokumen.pub/img/supersimple-chemistry-the-ultimate-bitesize-study-guide-1nbsped-1465493239-9781465493231-k-3312698.jpg)
SuperSimple Chemistry: The Ultimate Bitesize Study Guide [1 ed.] 1465493239, 9781465493231 - DOKUMEN.PUB
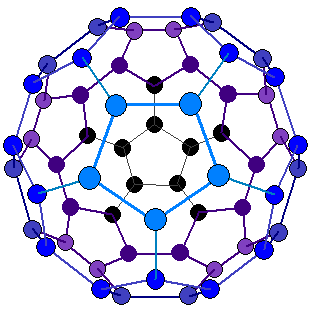
GCSE CHEMISTRY - What is Buckminsterfullerene? - What is the Structure of Buckminsterfullerene? - What is C60? - GCSE SCIENCE.
IGCSE Chemistry 2017: 1.50: Explain How the Structures of Diamond, Graphite and C60 Fullerene Influence their Physical properties, Including Electrical Conductivity and Hardness

Size does matter! Nanotubes may now power implants, straight from your bloodstream | by Sparrow | sparrow.science | Medium
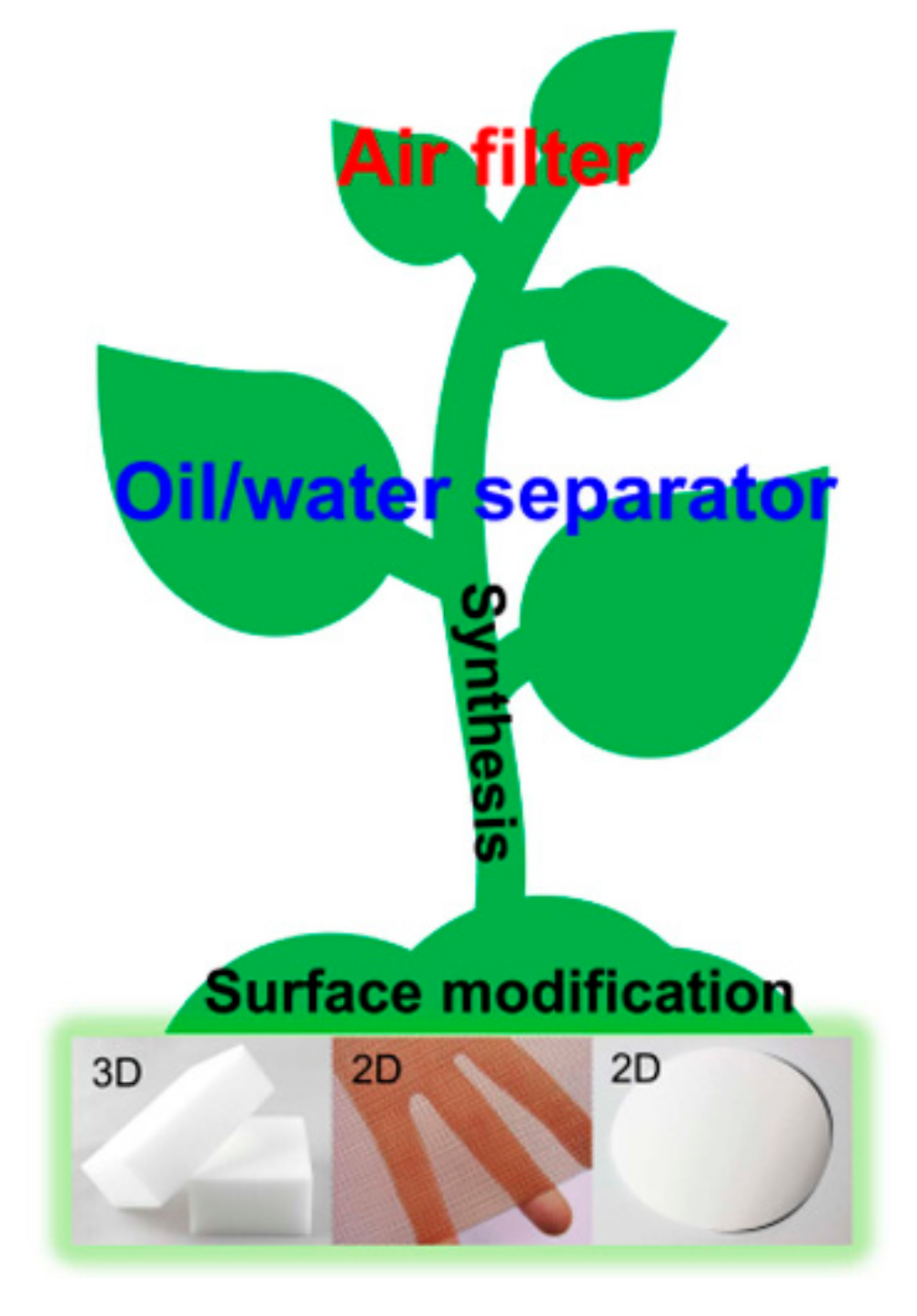
Materials | Free Full-Text | 2D and 3D Bulk Materials for Environmental Remediation: Air Filtration and Oil/Water Separation

J. Compos. Sci. | Free Full-Text | Electromagnetic Interference Shielding Effectiveness of Natural and Chlorobutyl Rubber Blend Nanocomposite

Graphene-based Nanomaterials in Fighting the Most Challenging Viruses and Immunogenic Disorders | ACS Biomaterials Science & Engineering