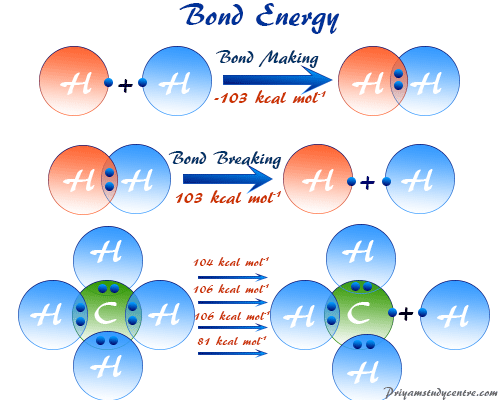
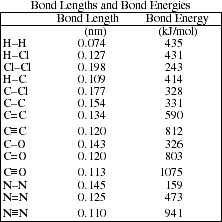
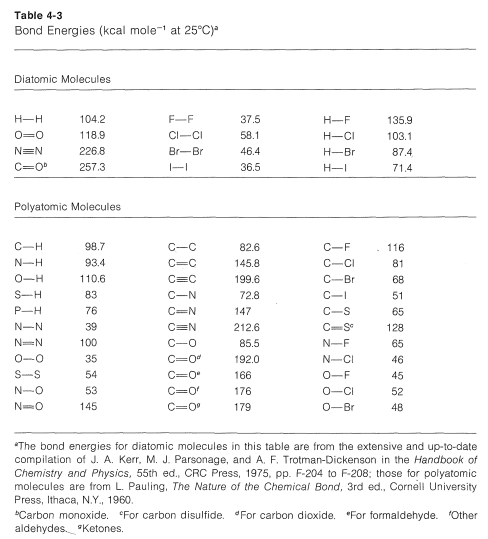
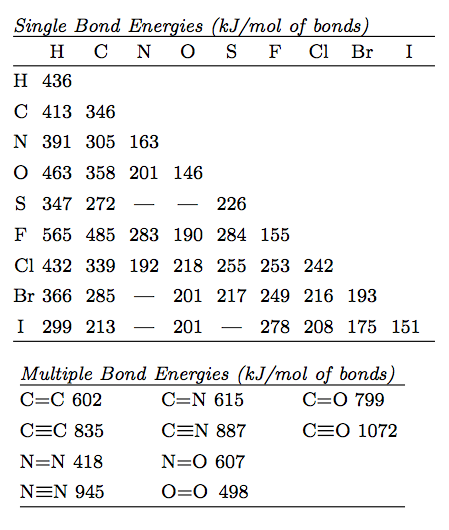
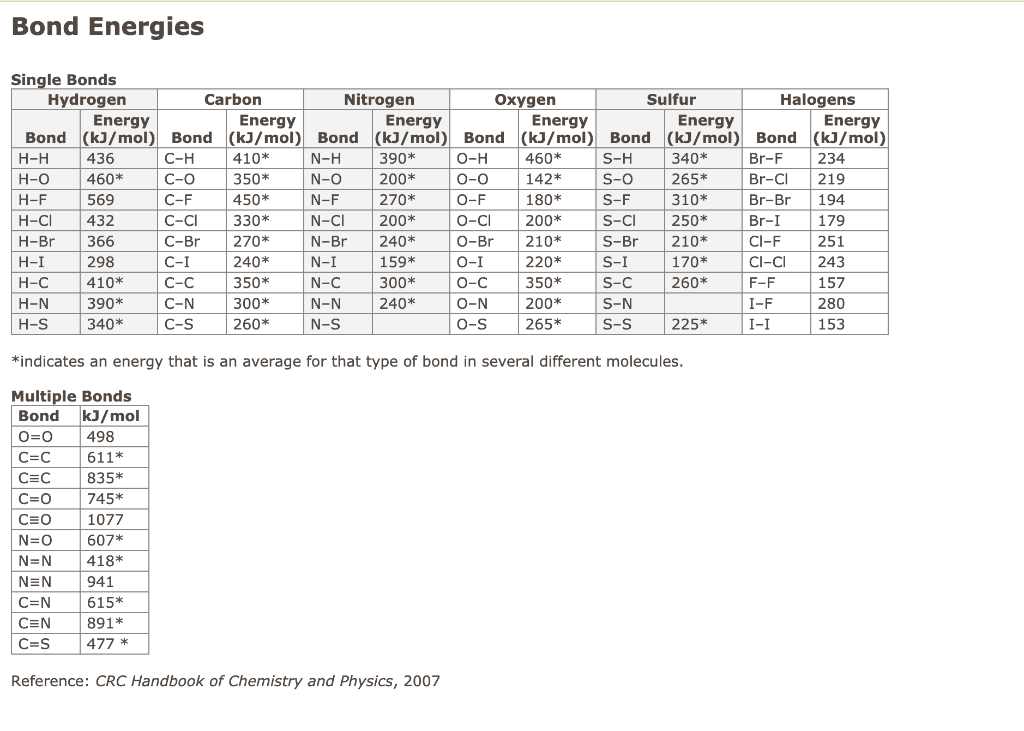
SOLVED: The average bond energy of a carbon-oxygen single bond is 358 kJ/mol. This means that 358 kilojoules of energy is needed to break a mole of carbon-oxygen single bonds. How much

SOLVED: 30. Which of the following molecules would have the largest bond dissociation energy associated with its carbon-oxygen bond? COClz Bs CO32 C COz D CH:OH E CO
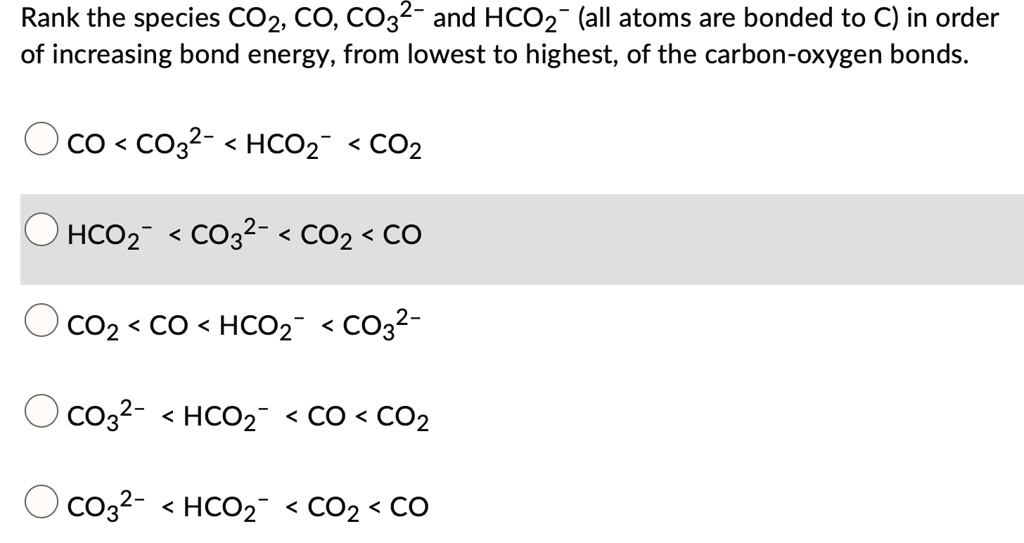
SOLVED: Rank the species CO2, CO, CO32- and HCO2" (all atoms are bonded to C) in order of increasing bond energy, from lowest to highest; of the carbon -oxygen bonds: CO CO32 -


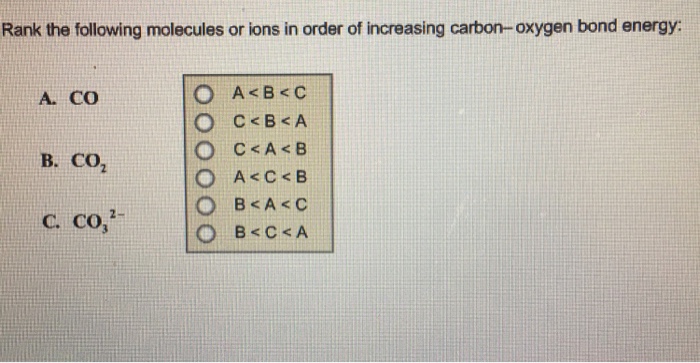

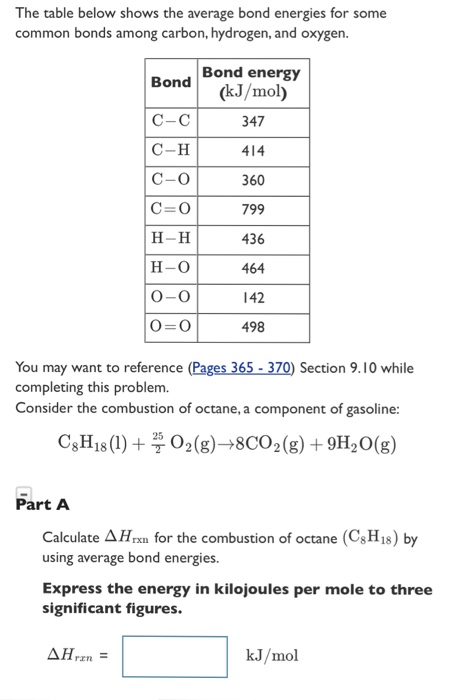



.PNG)