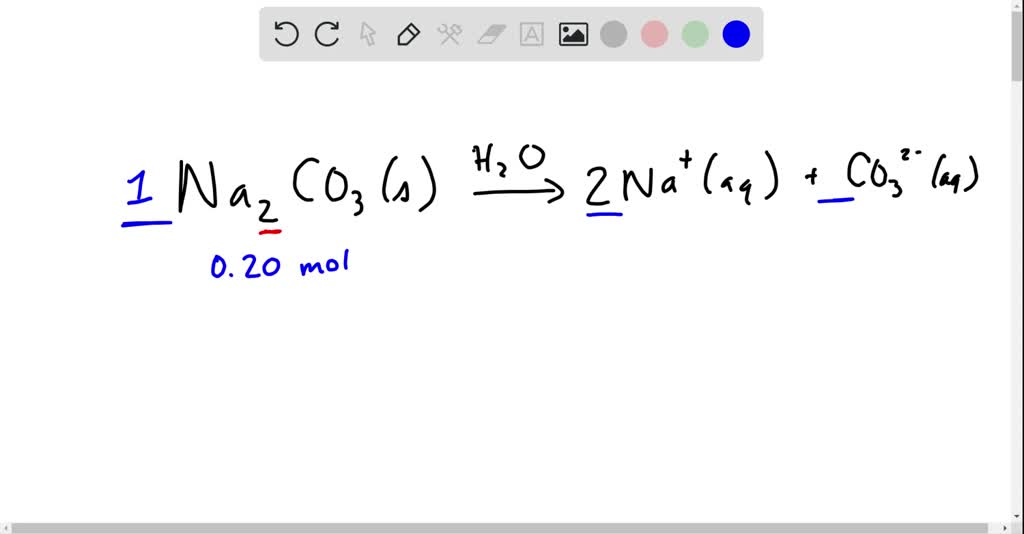
SOLVED:Write a balanced equation for the dissolution of sodium carbonate, Na2 CO3, in water. Find the number of moles of each ion produced when 0.20 mol of sodium carbonate dissolves. Then, find
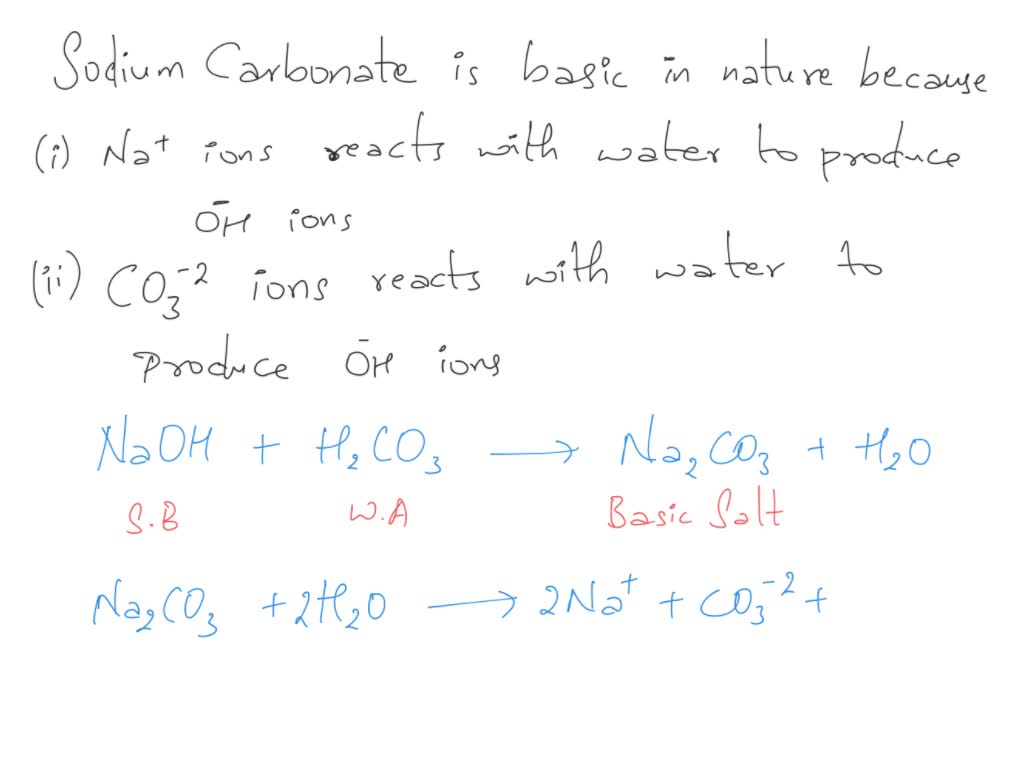
SOLVED: The best explanation for why sodium carbonate is basic is:sodium ions react with water to produce hydroxide ions ORCarbonate ions react with water to produce hydroxide ions

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride
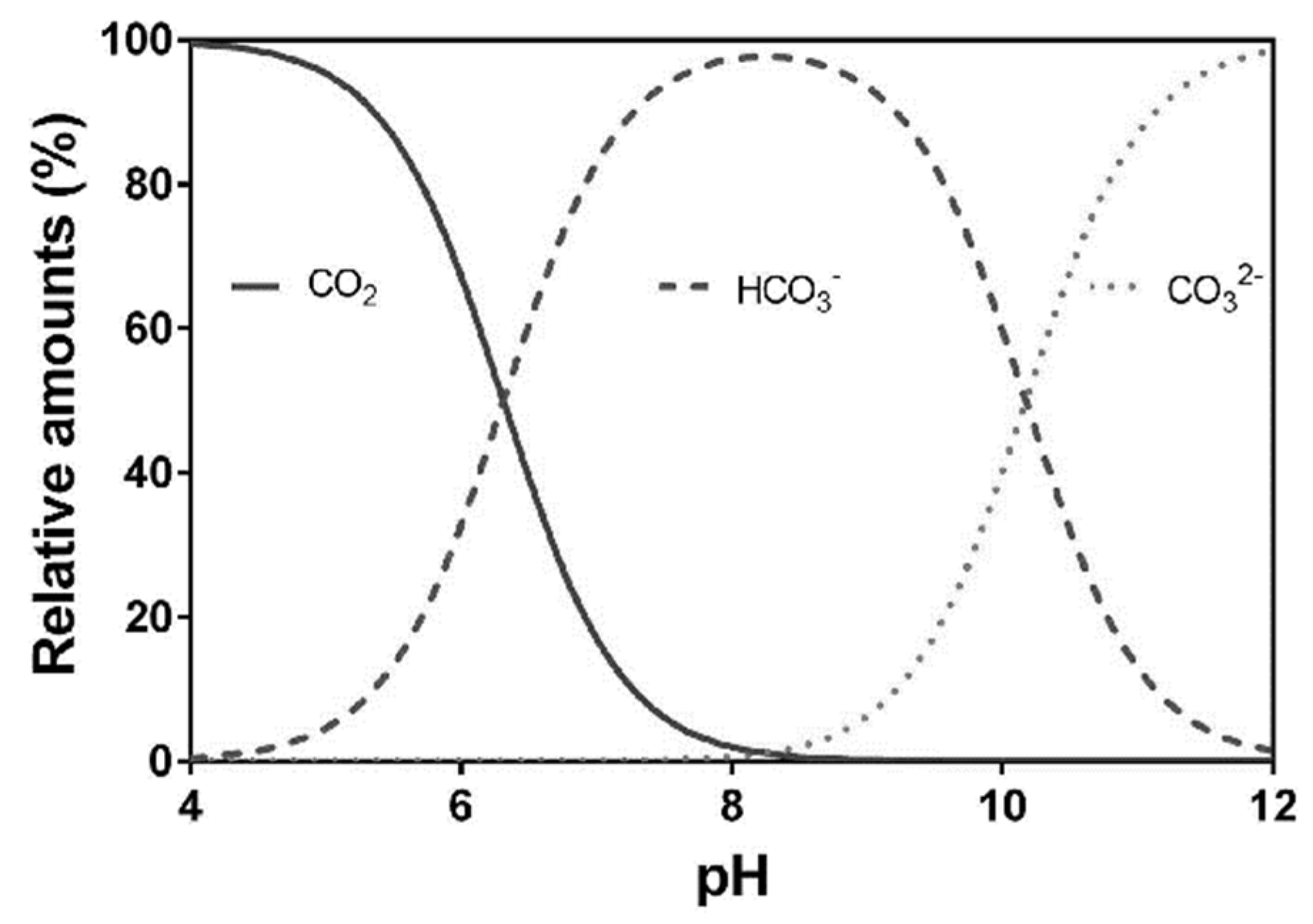
Water | Free Full-Text | Review of Techniques to Reduce and Prevent Carbonate Scale. Prospecting in Water Treatment by Magnetism and Electromagnetism
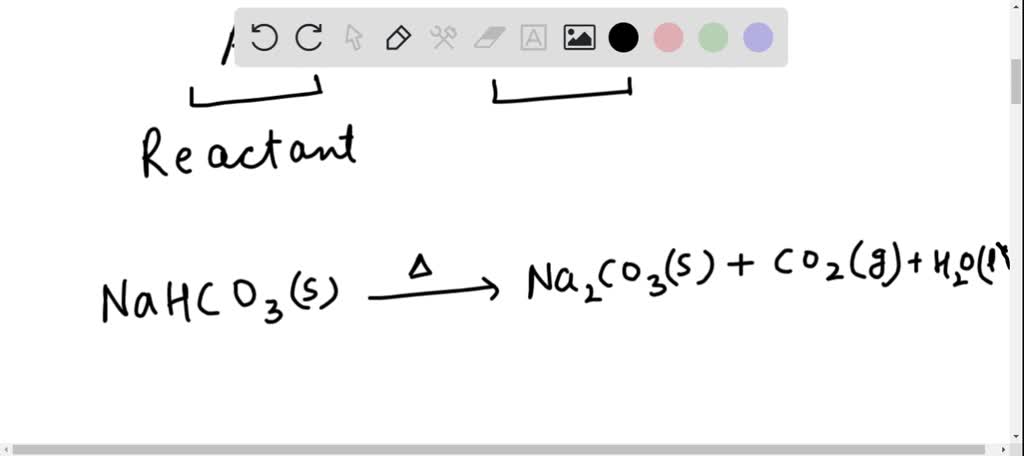
SOLVED: Write the balanced chemical reaction, including phase labels, for this reaction: "solid sodium bicarbonate decomposes into solid sodium carbonate, gaseous carbon dioxide, and liquid water."
Write the balanced chemical equations for the following reactions: i. Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water - Sarthaks eConnect | Largest Online Education Community