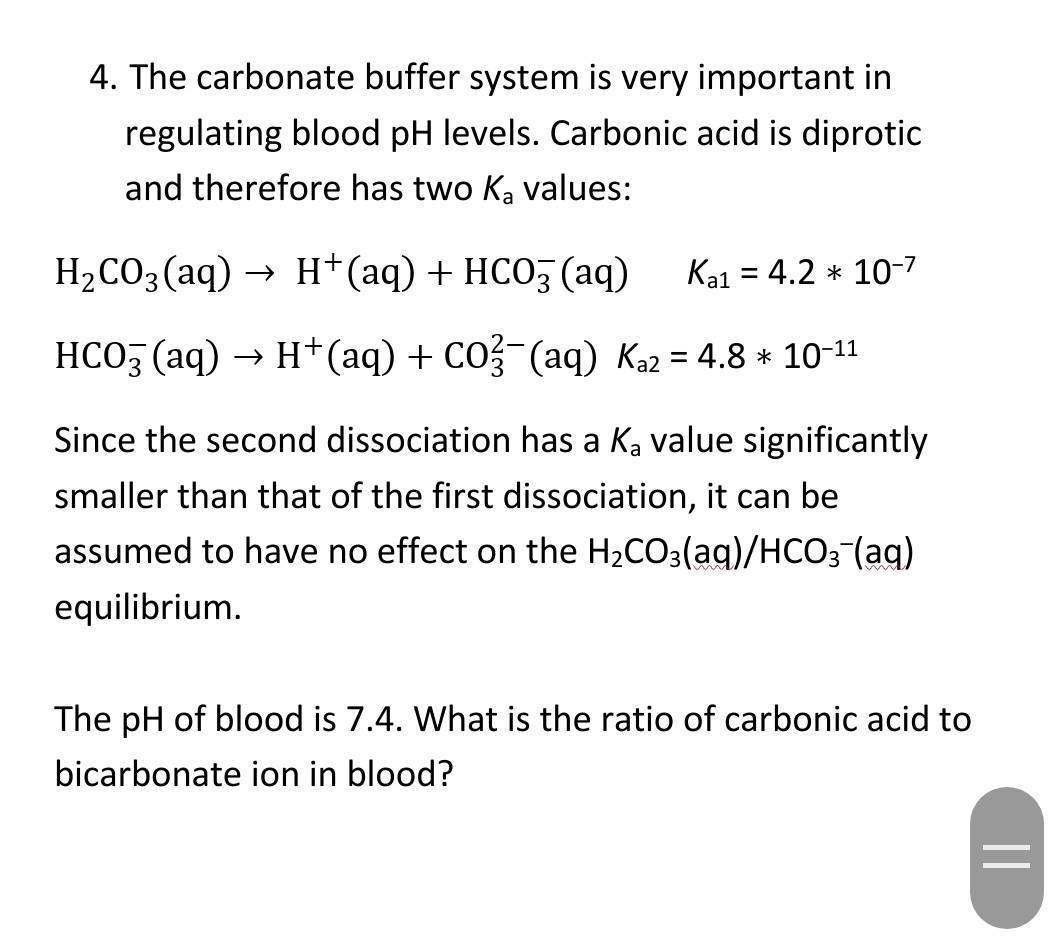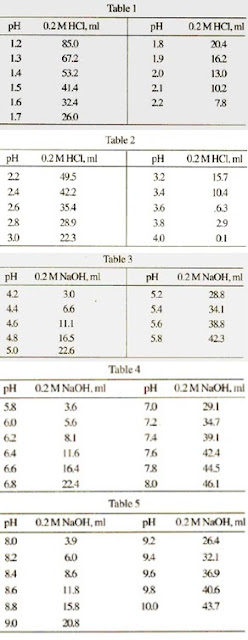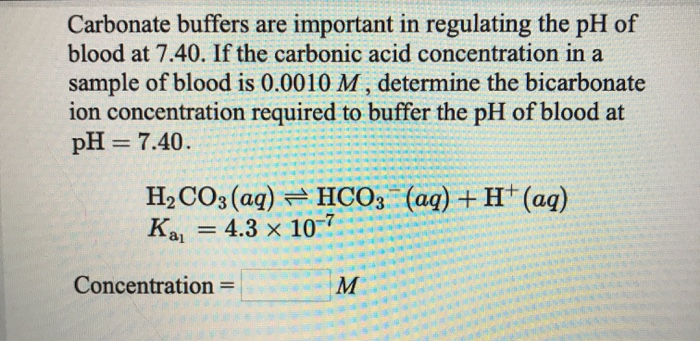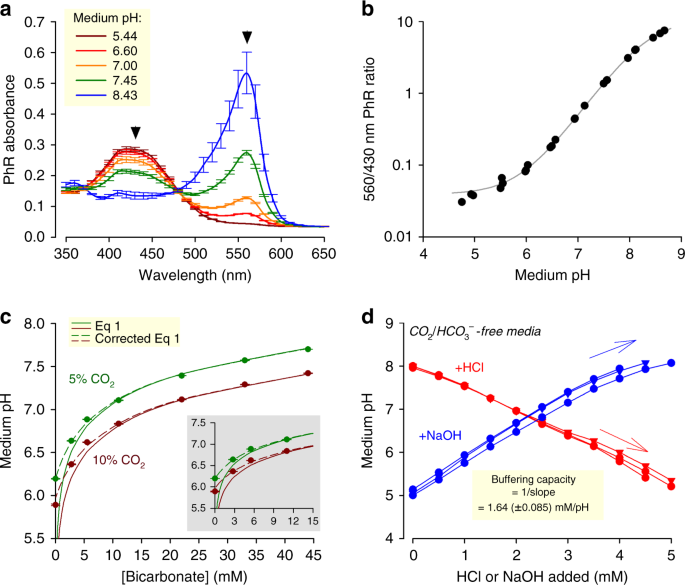
Evidence-based guidelines for controlling pH in mammalian live-cell culture systems | Communications Biology

H/EGFP 1.5 μM in a 20 mM pH 7 phosphate buffer (×) and pH 10.4 (◊),... | Download Scientific Diagram

SOLVED: You need to prepare carbonate buffer of pH 10.00 for studying the effects of acid rain on limestone rich soils. The K for bicarbonate (HCO: ) is 4.7 x 10-4. How

An enviromental chemist needs a carbonate buffer of pH 10 to study the effects of the acidification of limestone - rich soils.How many grams of Na2CO3 must be added to 1.5 L
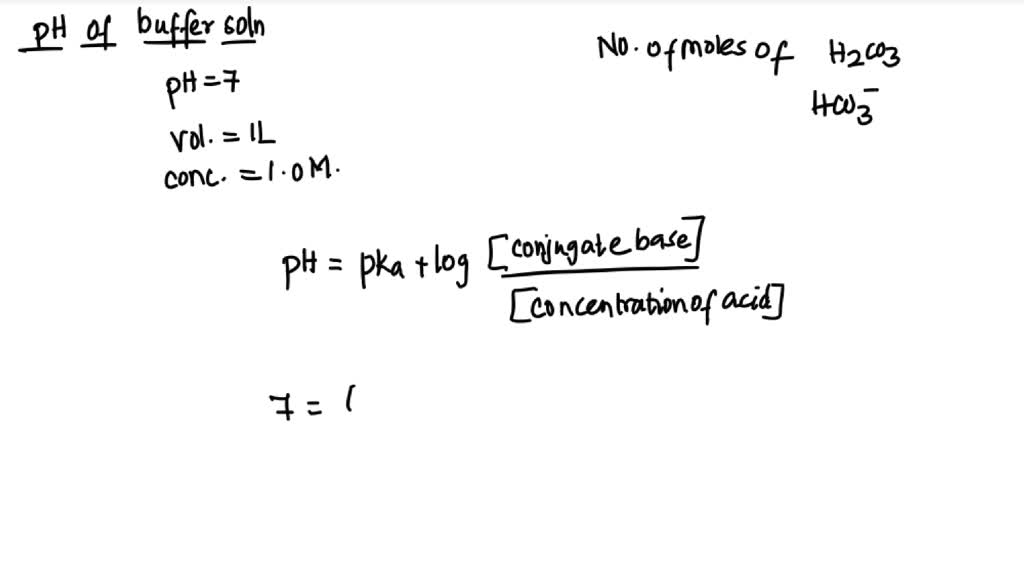
SOLVED: If the pH of 1 liter of a 1.0 M carbonate buffer is 7.0,what is actual number of moles of HzCO3 and HCO3 ? (pK 6.,37) moles ofHZCO; 0.86 0.81 0.76 0.19 0,14 moles of HCO3 674 p.19 624 0.81 6.86 01 0 0 Qiv Ov




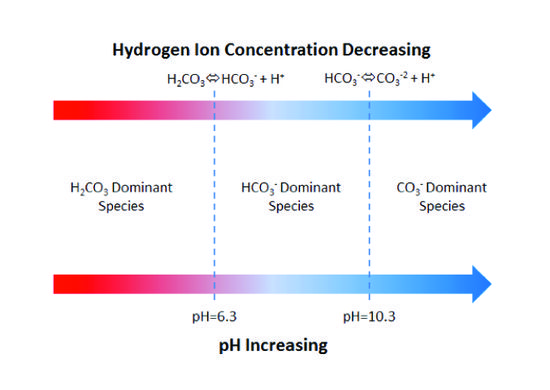
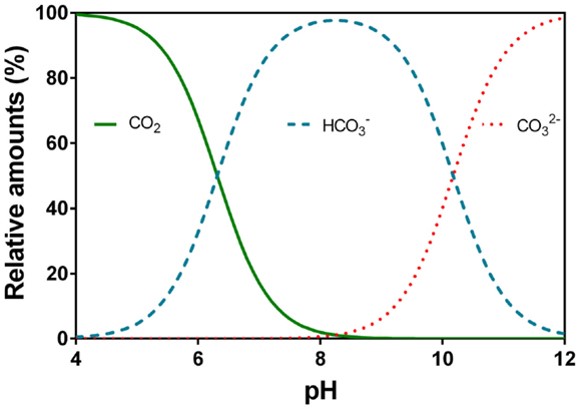





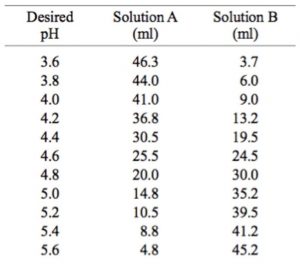


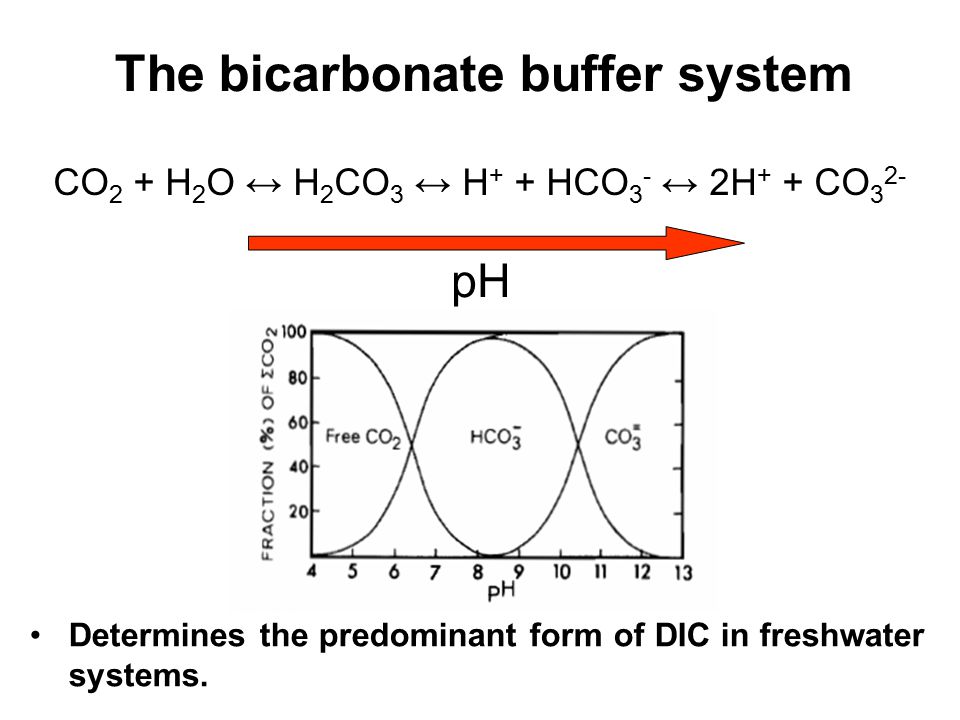
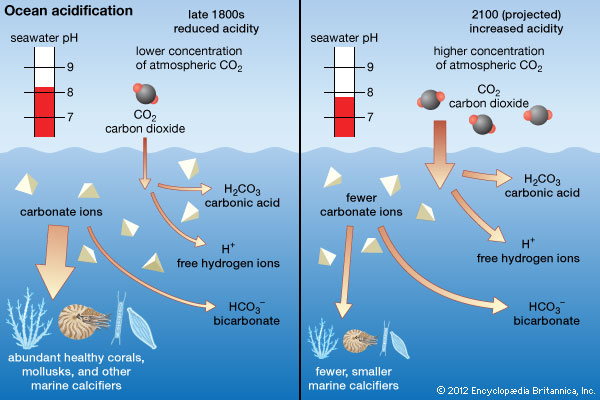
![PDF] A sodium carbonate-bicarbonate buffer for alkaline phosphatases. | Semantic Scholar PDF] A sodium carbonate-bicarbonate buffer for alkaline phosphatases. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/aaa98c1f9de4c398b6a057bf4936ea76736034c7/1-Table1-1.png)