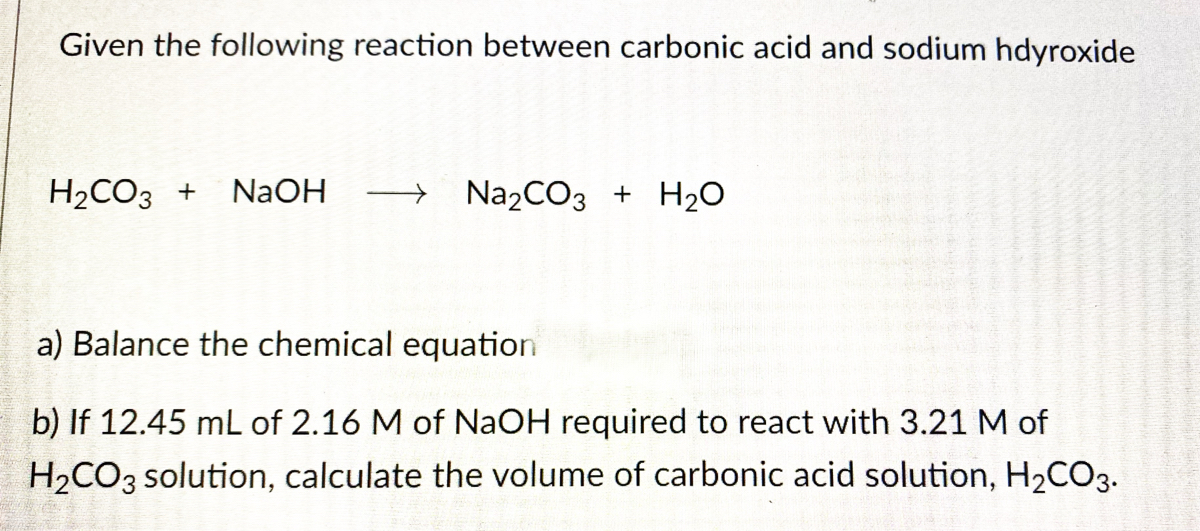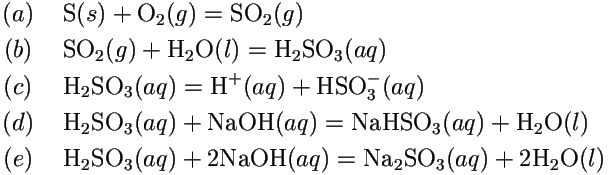
Experimental study on capture of carbon dioxide and production of sodium bicarbonate from sodium hydroxide

Give reason for each of the following:Aqueous solutions of carbonates of alkali metals are alkaline in nature?

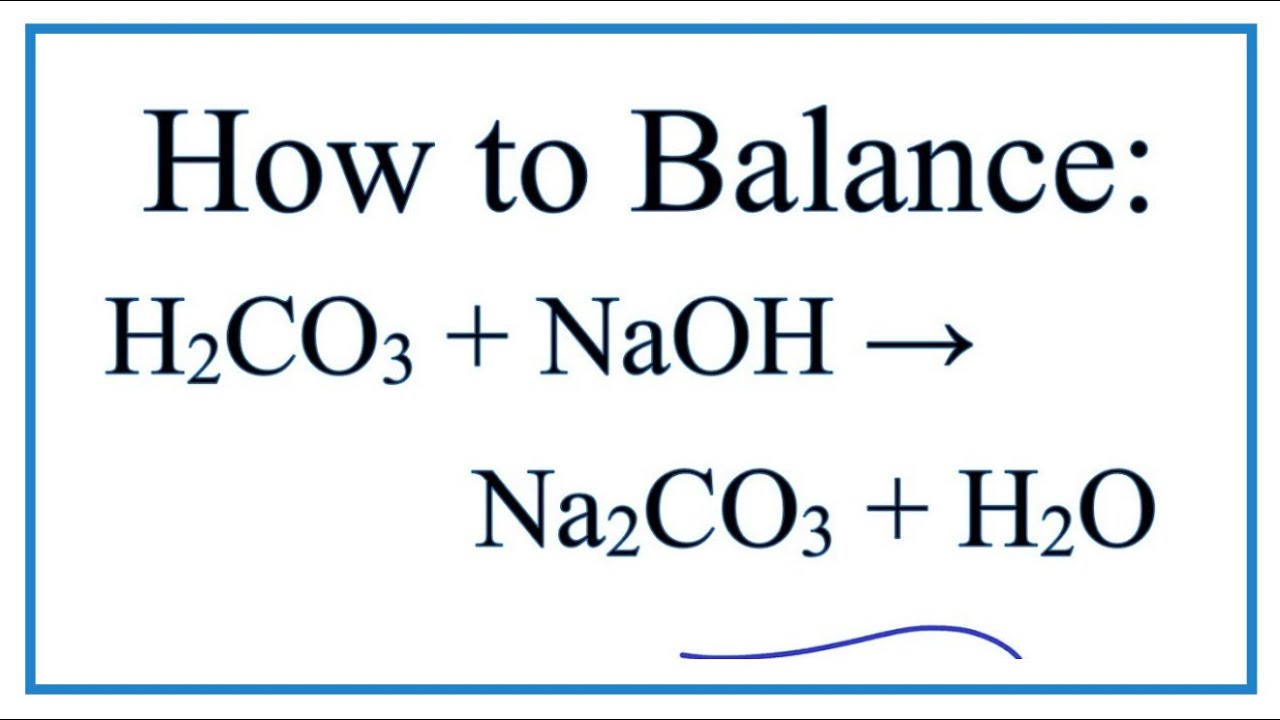
It takes 60 mL of 0.20 M of sodium hydroxide (NaOH) to neutralize 25 mL of carbonic acid (H2CO3) for the - Brainly.com

Question: Write the chemical reaction when lithium hydroxide is mixed with carbonic acid. Step 1: write out the reactants LiOH (aq) + H 2 CO 3 (aq) - ppt download

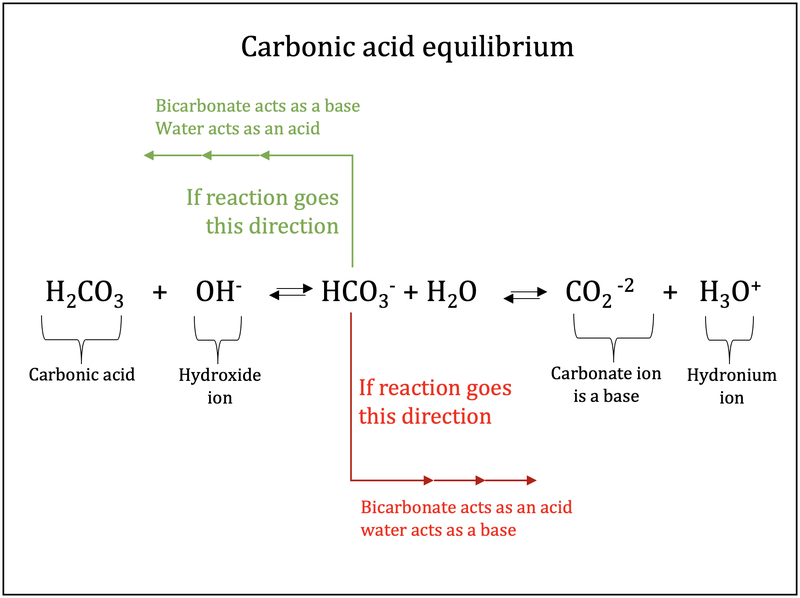
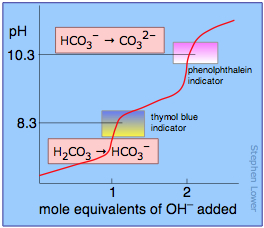
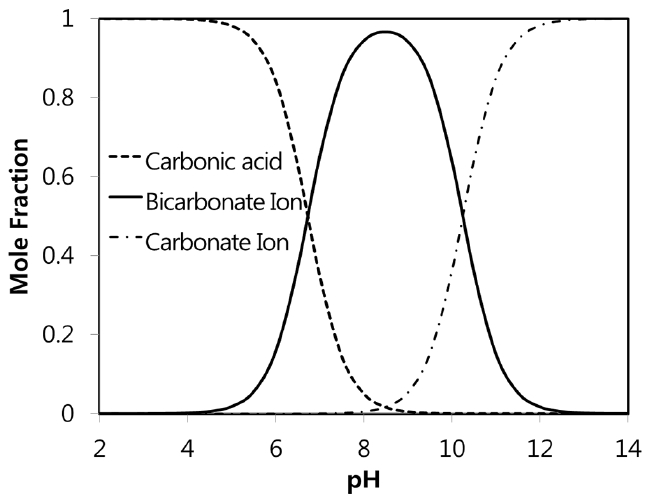
physical chemistry - Which make HCO3- to show two pH values at two scenarios? - Chemistry Stack Exchange

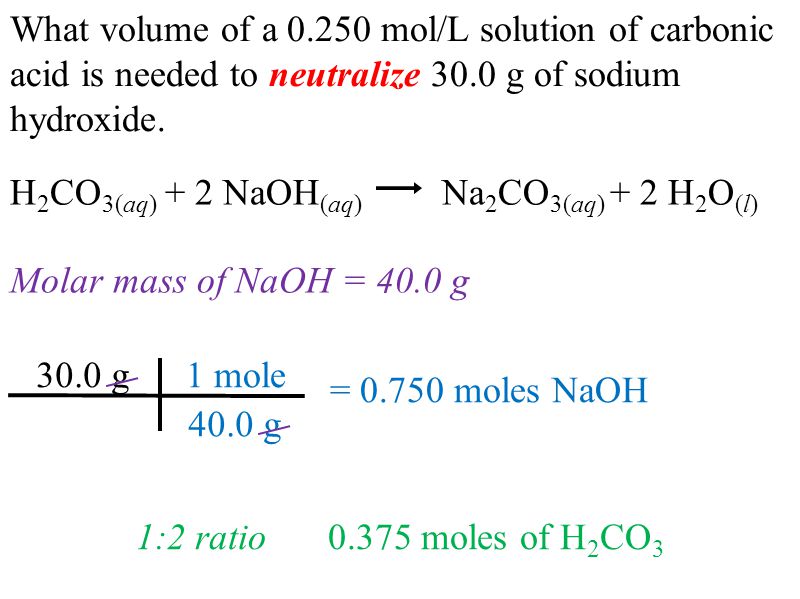
OneClass: a) Write a balanced molecular equation for the complete neutralization of the diprotic acid...

physical chemistry - Which make HCO3- to show two pH values at two scenarios? - Chemistry Stack Exchange

What Is Carbonic acid, magnesium salt (1:1), mixt. with magnesium hydroxide (Mg(OH)2), hydrate, Cas No 39409-82-0 Guide - ECHEMI