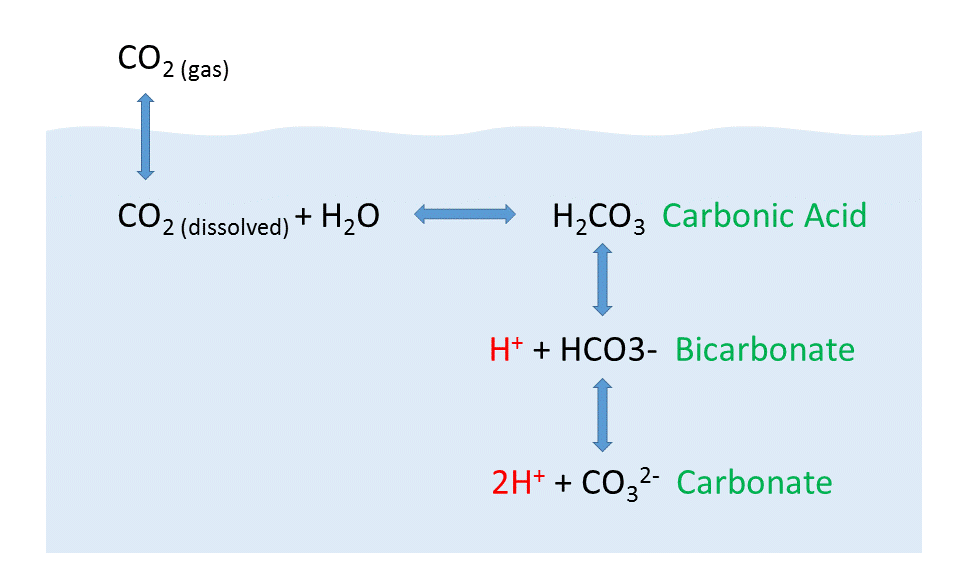
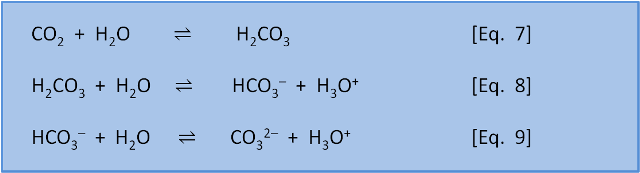
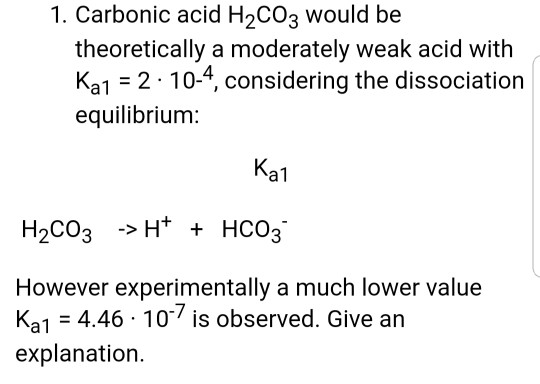
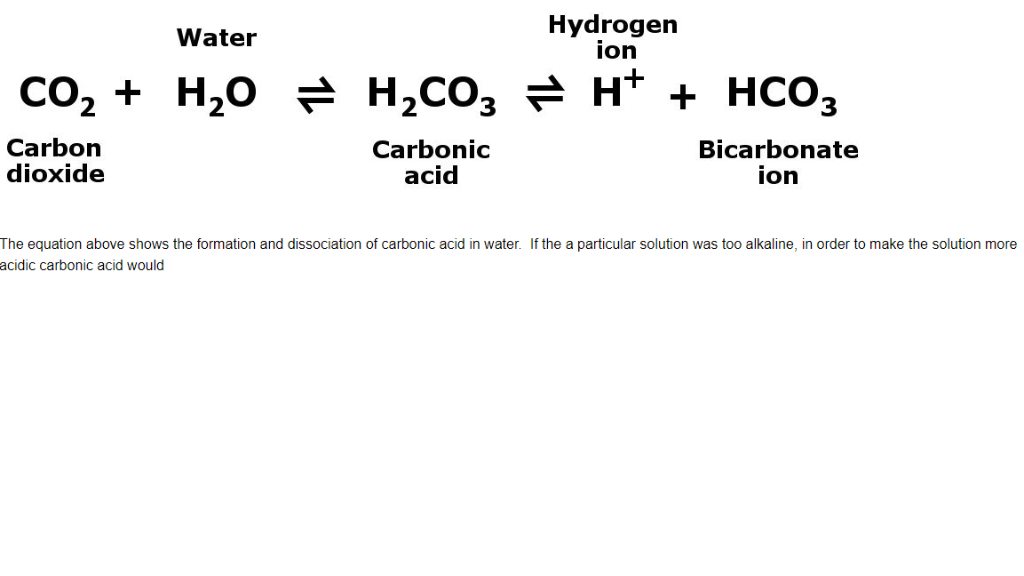
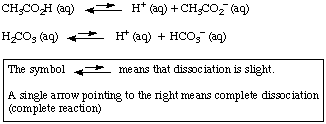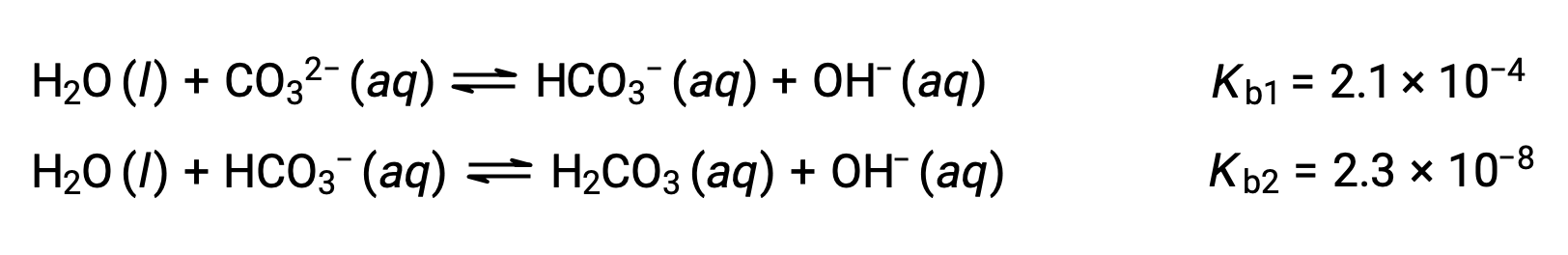Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora

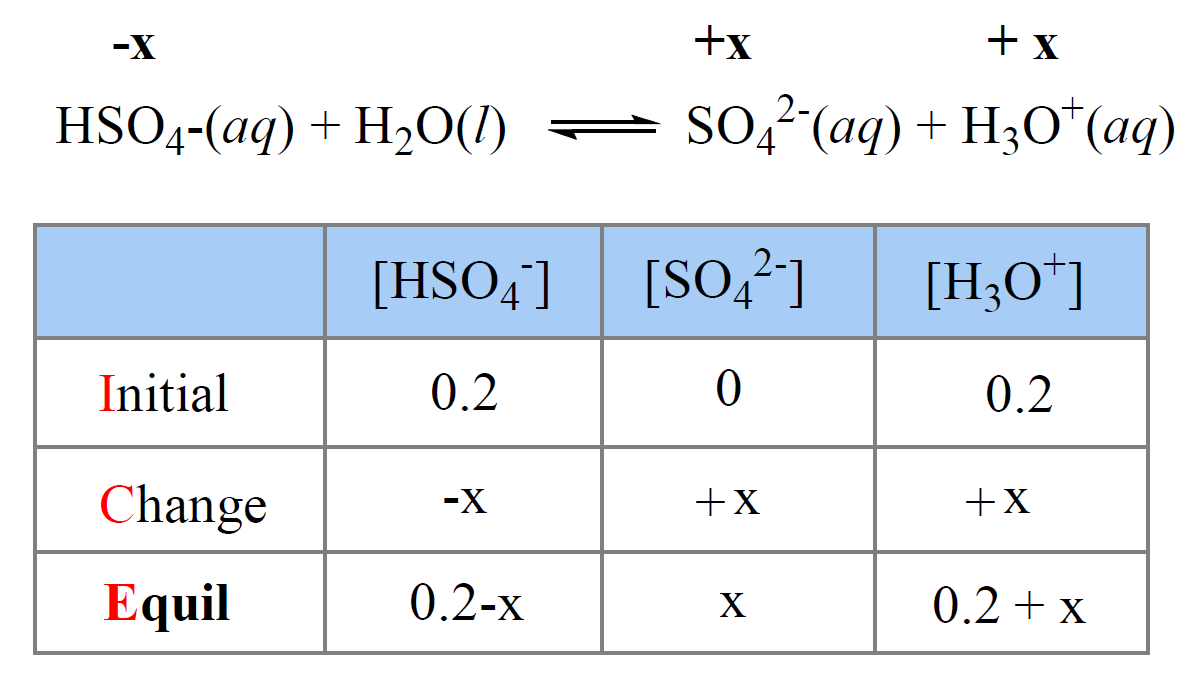
Acids and Bases Review/Equilibrium reversible reaction: R P and R P Acid dissociation is a reversible reaction and is said to be in equilibrium. H 2 SO. - ppt download
![SOLVED: The equilibrium constant for the first step dissociation of Carbonic Acid could be written as Point) Note Chemical formula of Carbonic Acid is HzCO3 [H - 1+co; | [H-COs] Kal H - SOLVED: The equilibrium constant for the first step dissociation of Carbonic Acid could be written as Point) Note Chemical formula of Carbonic Acid is HzCO3 [H - 1+co; | [H-COs] Kal H -](https://cdn.numerade.com/ask_images/4aa036e142eb4832ae078b434fceb3cf.jpg)
SOLVED: The equilibrium constant for the first step dissociation of Carbonic Acid could be written as Point) Note Chemical formula of Carbonic Acid is HzCO3 [H - 1+co; | [H-COs] Kal H -