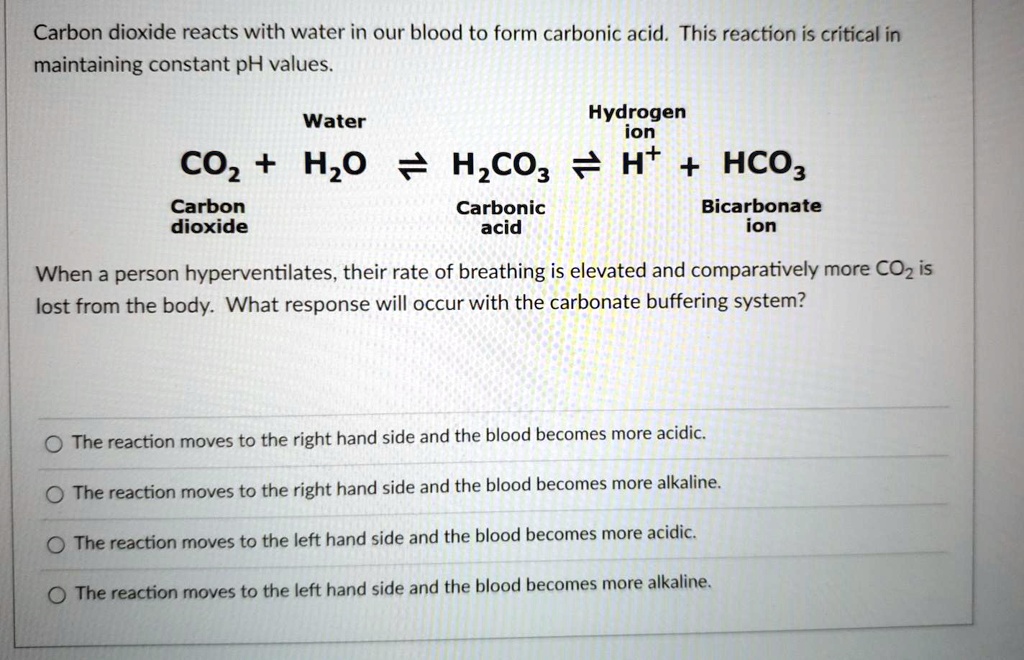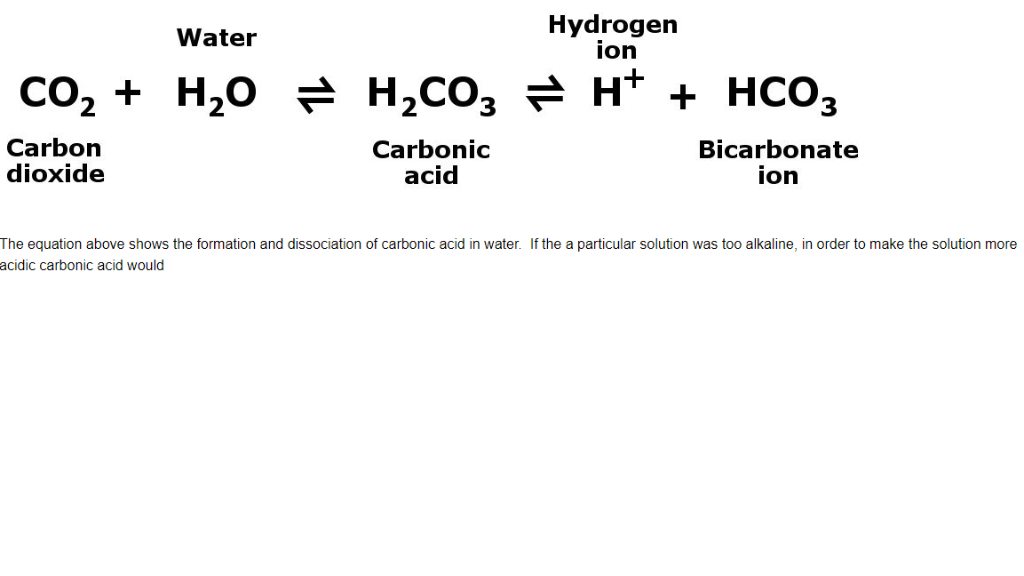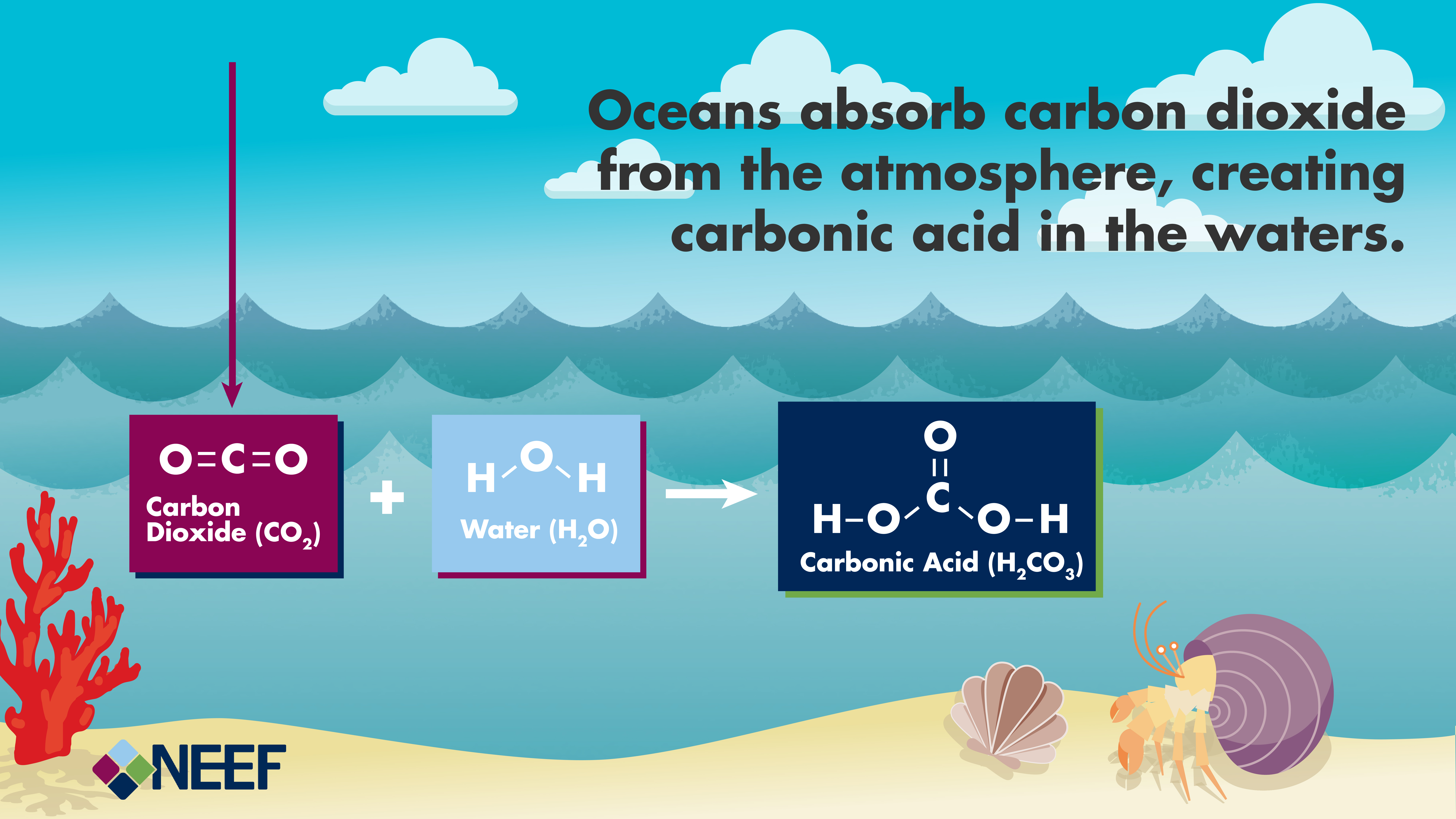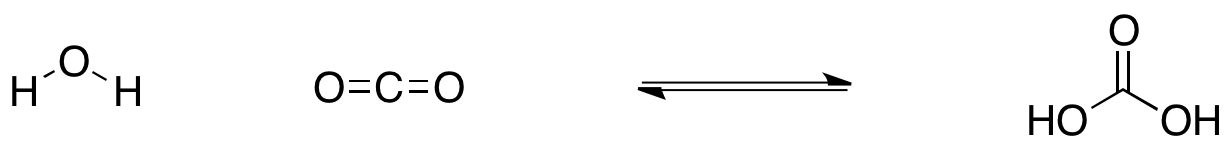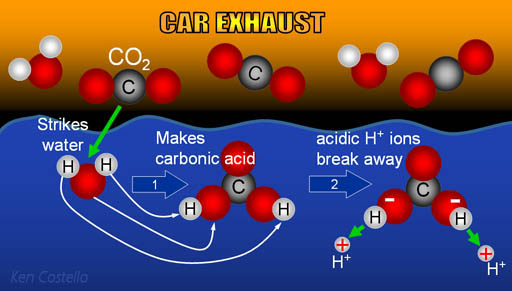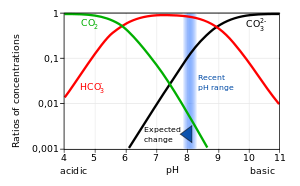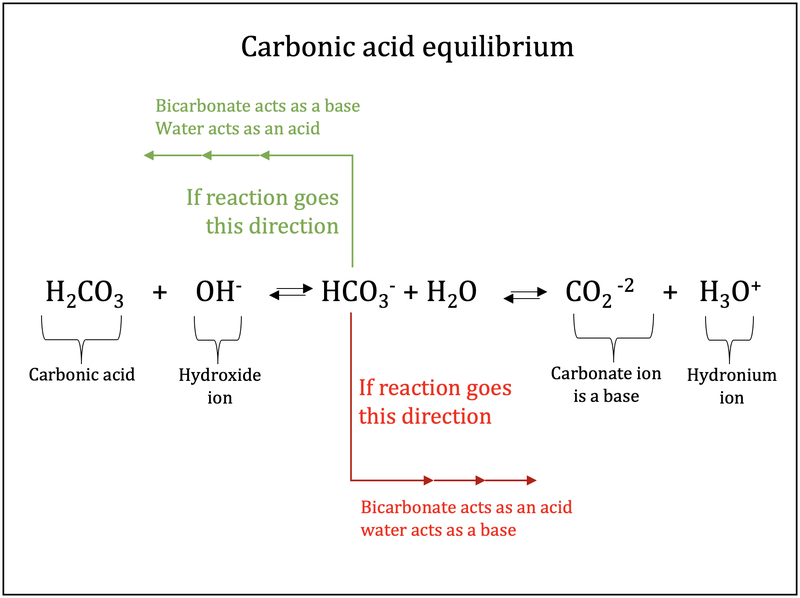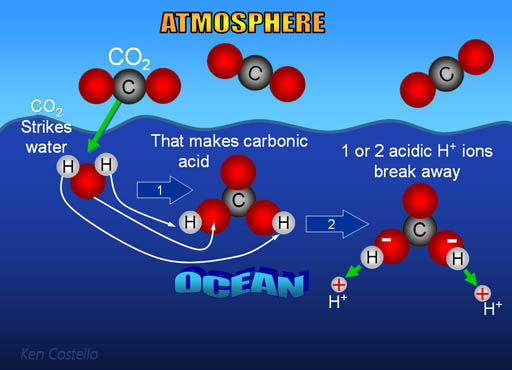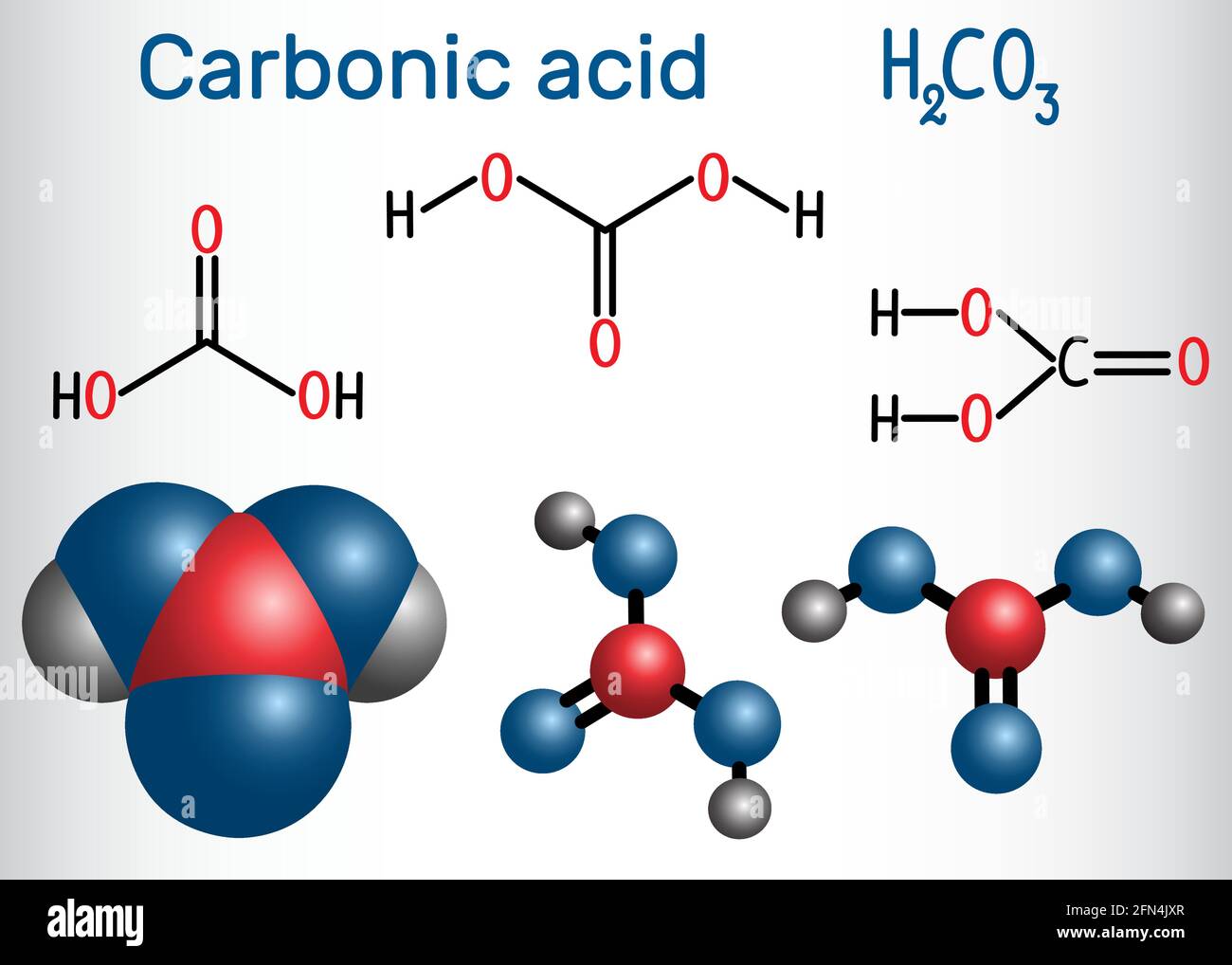
Carbonic acid (H2CO3) molecule . It is also solution of carbon dioxide in water (carbonated water). Structural chemical formula and molecule model. V Stock Vector Image & Art - Alamy
Visualization of the shortest routes for the decomposition of isolated... | Download Scientific Diagram
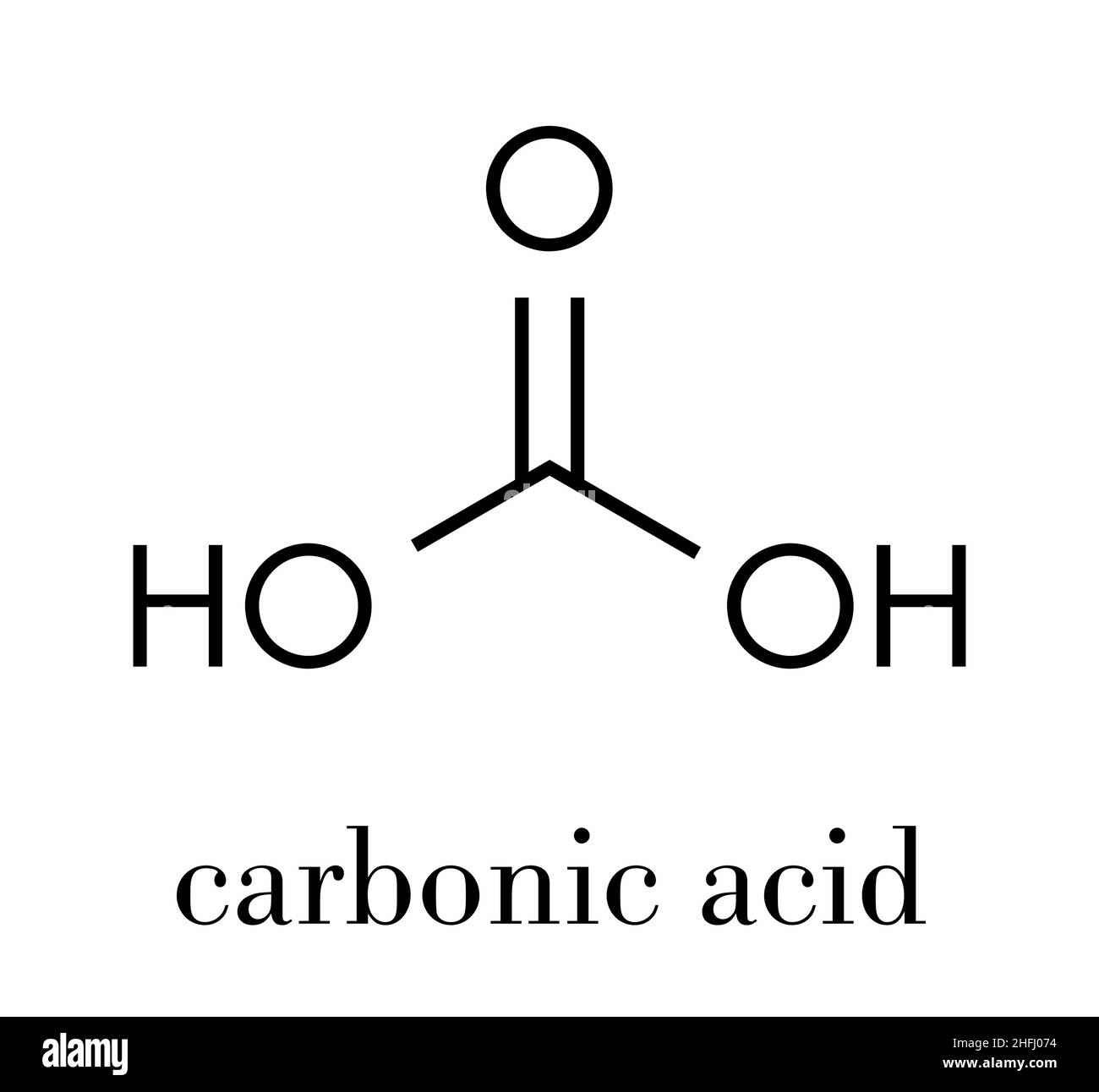
Carbonic acid molecule. Formed when carbon dioxide is dissolved in water (carbonated water). Skeletal formula Stock Vector Image & Art - Alamy
What is the most likely mechanism for the formation of carbonic acid from water and carbon dioxide? - Quora
Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora