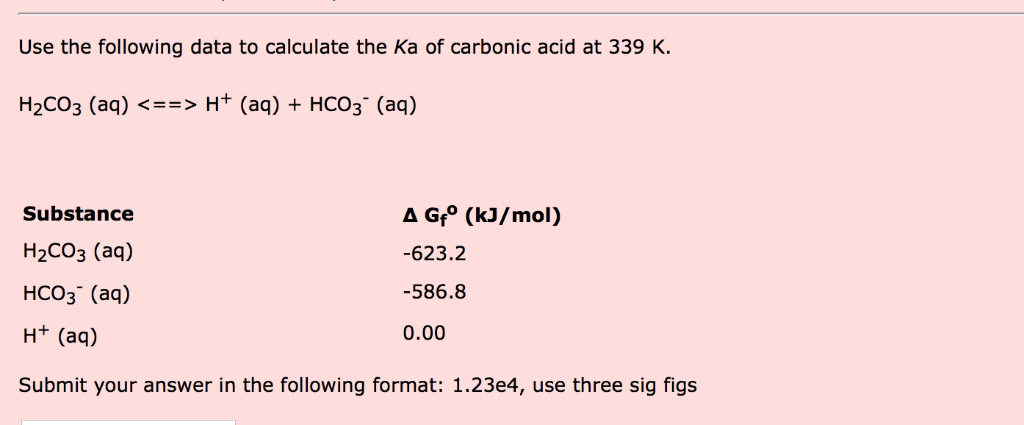
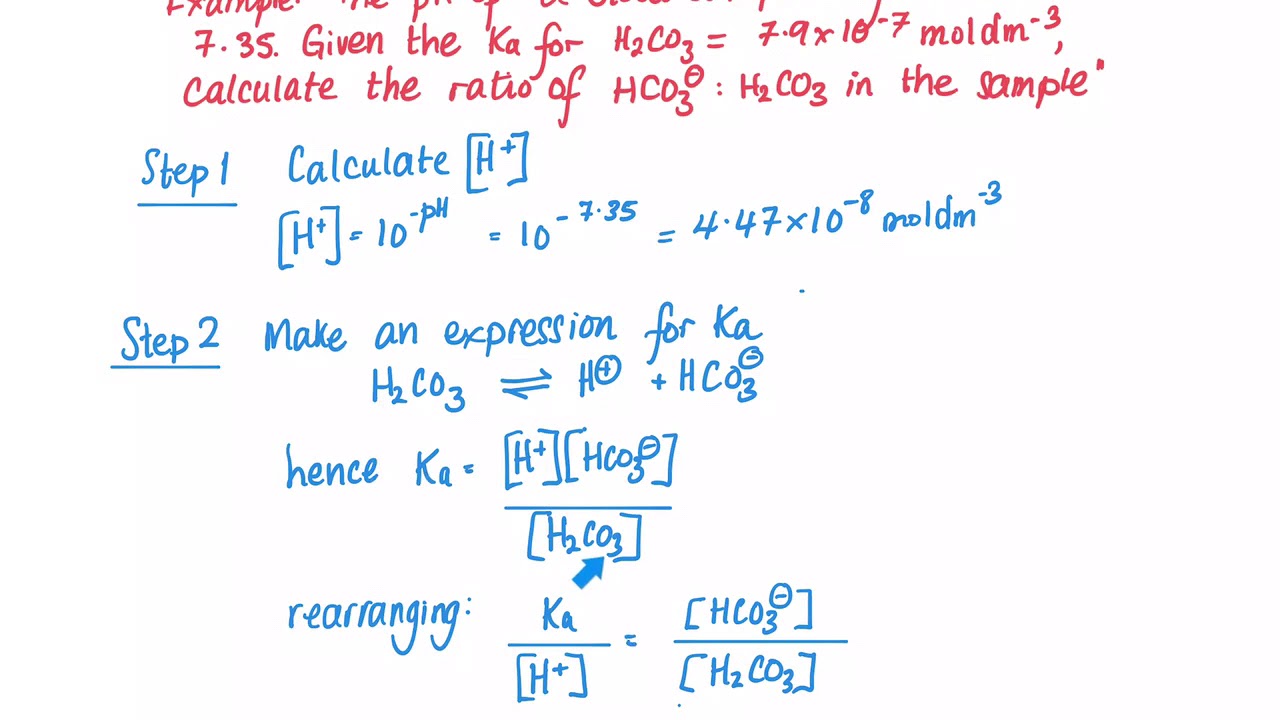
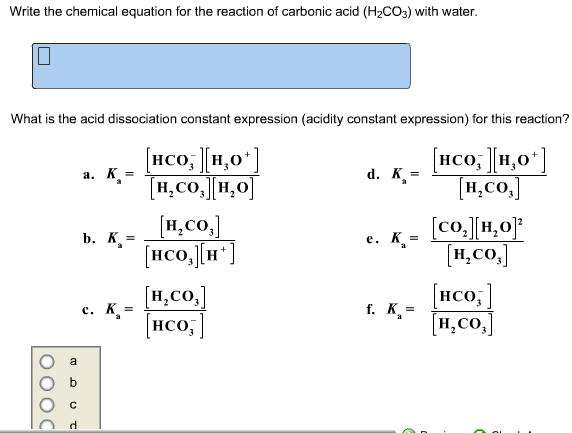
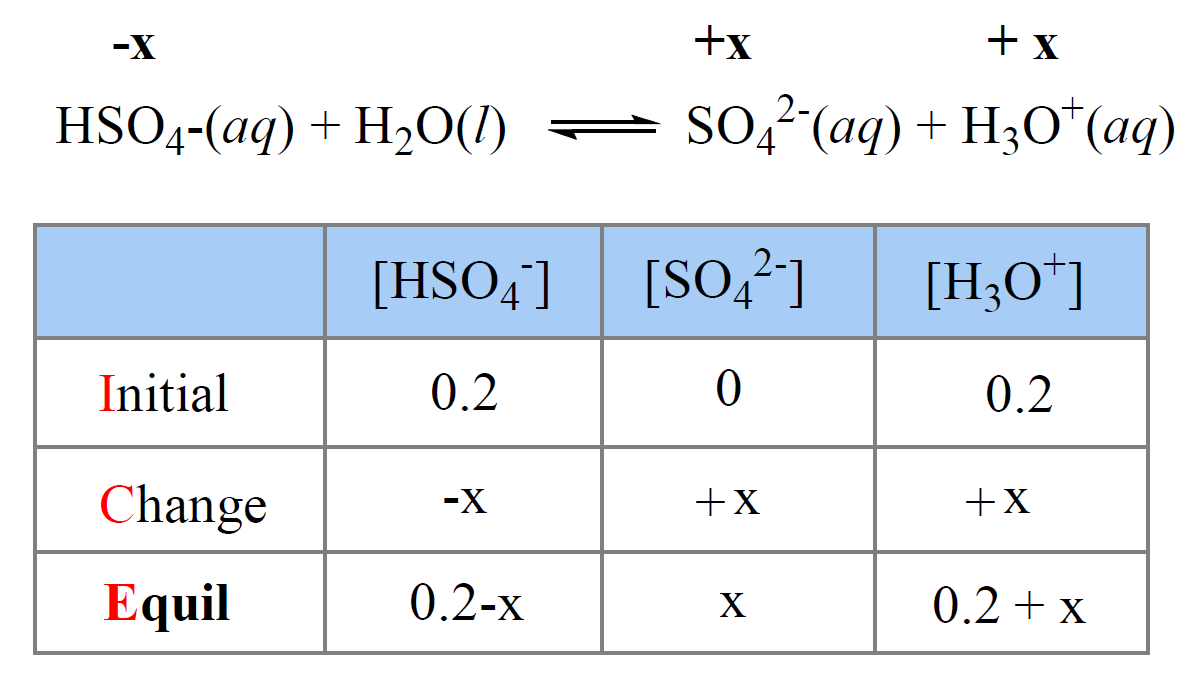
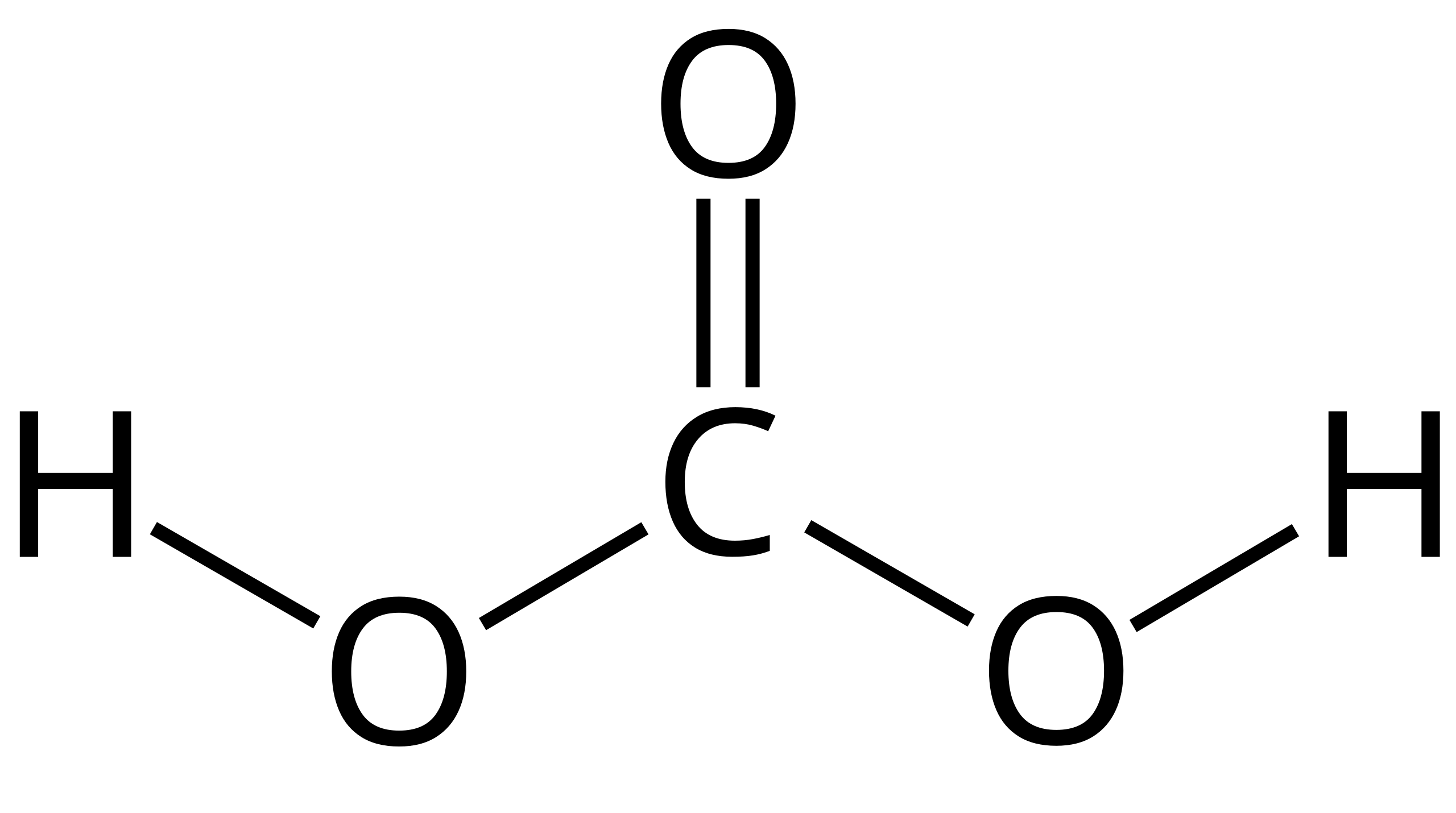
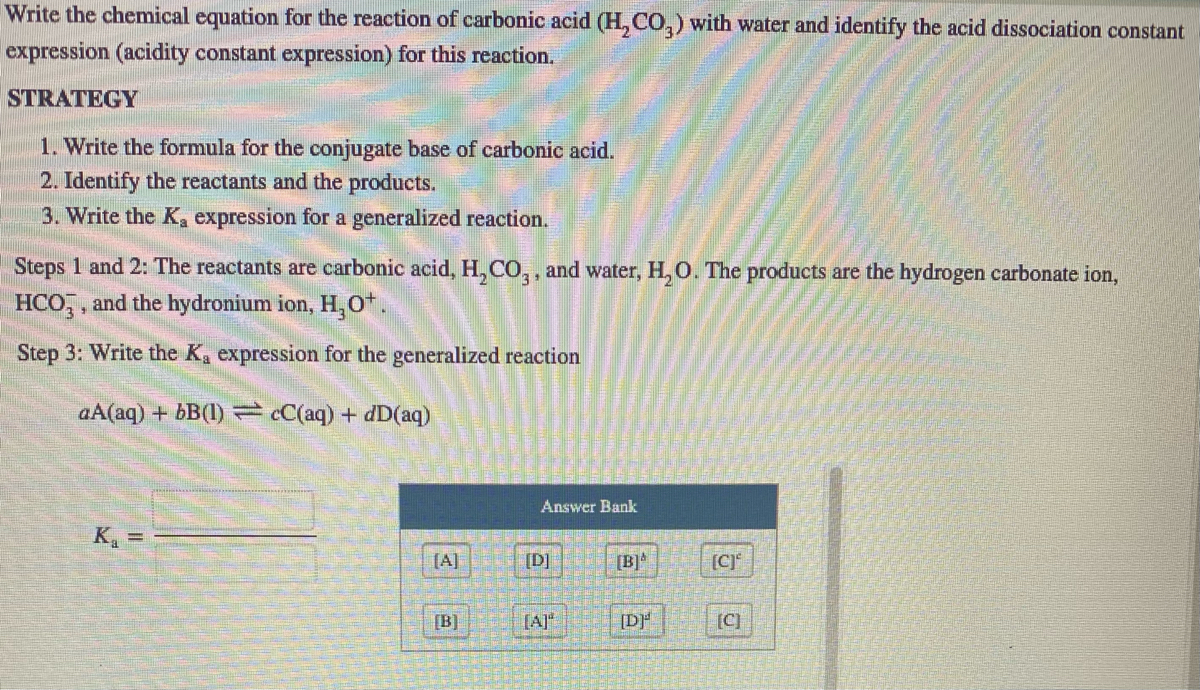
OneClass: Consider the acid dissociation behavior of carbonic acid, H2CO3. pKa 6.351 pka2 10.329 HO C...

Calculate the pH (nearest integer) of 0.010M NaHCO3 solution. K1 = 4.5 × 10^-7 and K2 = 4.7 × 10^-11 for carbonic acid.
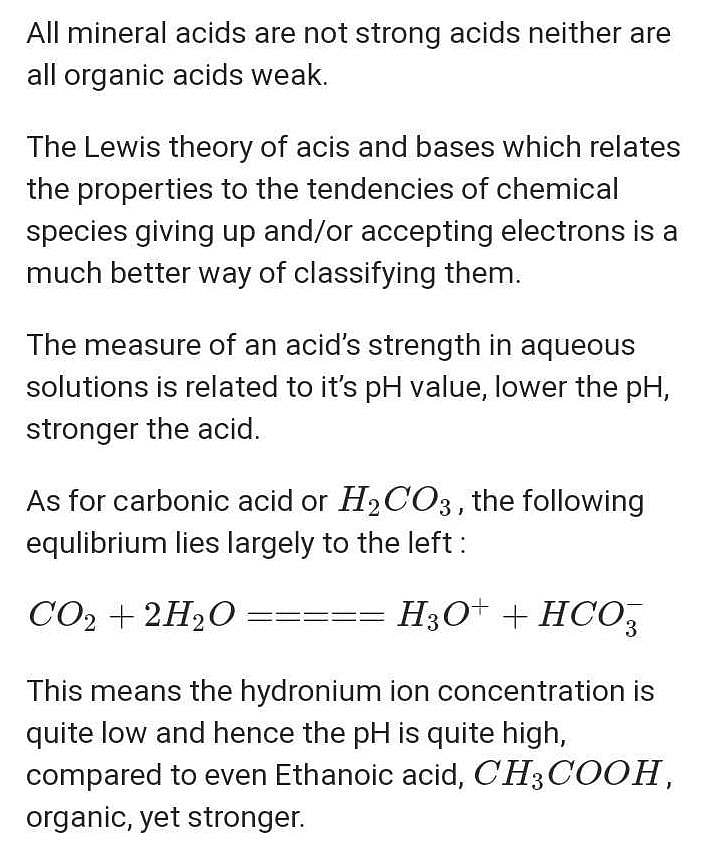
Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora
SOLVED: If the carbonic acid (H2CO3) concentration in a sample of blood is 0.00125 M, determine the bicarbonate ion (HCO3-) concentration required to buffer the pH of blood at pH = 7.40.
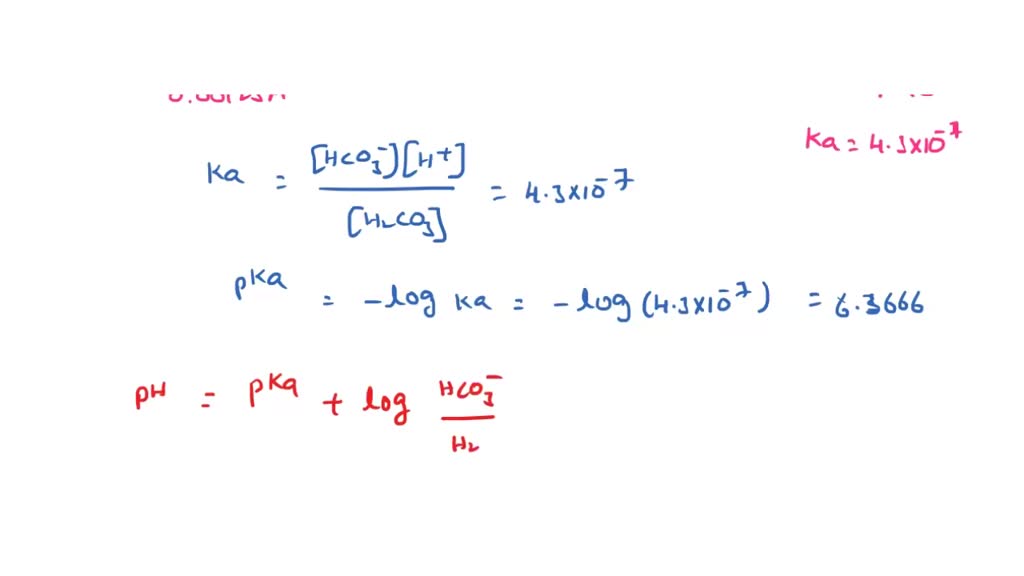
![SOLVED: The equilibrium constant for the first step dissociation of Carbonic Acid could be written as Point) Note Chemical formula of Carbonic Acid is HzCO3 [H - 1+co; | [H-COs] Kal H - SOLVED: The equilibrium constant for the first step dissociation of Carbonic Acid could be written as Point) Note Chemical formula of Carbonic Acid is HzCO3 [H - 1+co; | [H-COs] Kal H -](https://cdn.numerade.com/ask_images/4aa036e142eb4832ae078b434fceb3cf.jpg)
SOLVED: The equilibrium constant for the first step dissociation of Carbonic Acid could be written as Point) Note Chemical formula of Carbonic Acid is HzCO3 [H - 1+co; | [H-COs] Kal H -