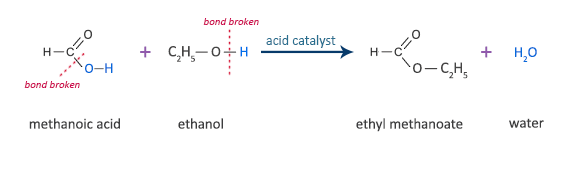
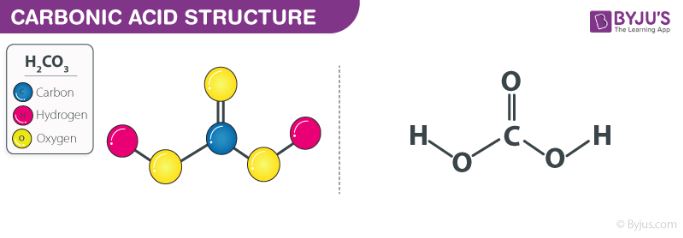
Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora
Arrange in increasing order of the strenght of acids Acetic acid, carbonic acid, sulphuric acid. (with reason)

Catalytic Reductive Transformations of Carboxylic and Carbonic Acid Derivatives Using Molecular Hydrogen | ACS Catalysis

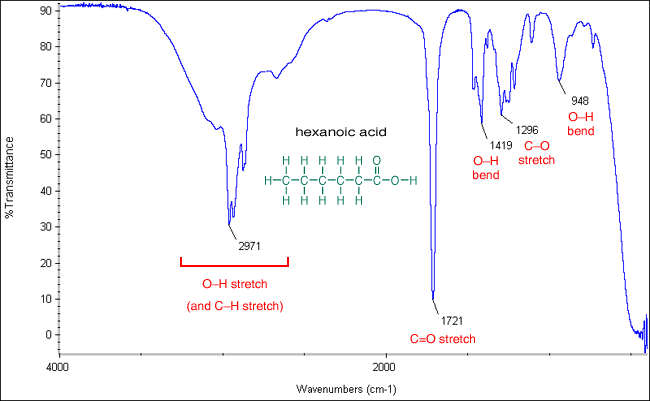
pKa Ka data factors affecting Acidic reactions of carboxylic acids with metals oxides hydroxides carbonates hydrogencarbonate test advanced A level organic chemistry revision notes doc brown

Catalytic transformation of functionalized carboxylic acids using multifunctional rhenium complexes | Scientific Reports

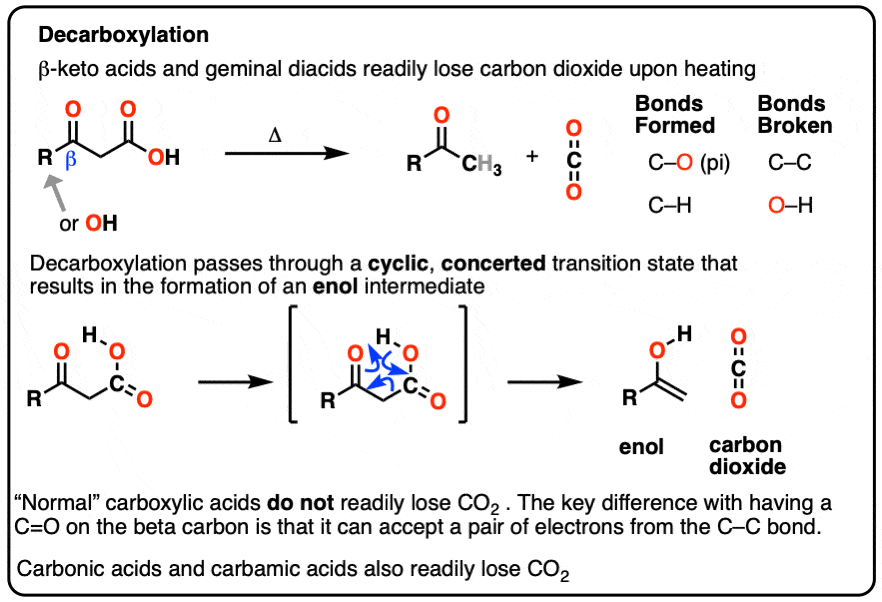
Protonated Carbonic Acid and Reactive Intermediates in the Acidic Decarboxylation of Indolecarboxylic Acids | The Journal of Organic Chemistry

Protonated Carbonic Acid and Reactive Intermediates in the Acidic Decarboxylation of Indolecarboxylic Acids | The Journal of Organic Chemistry