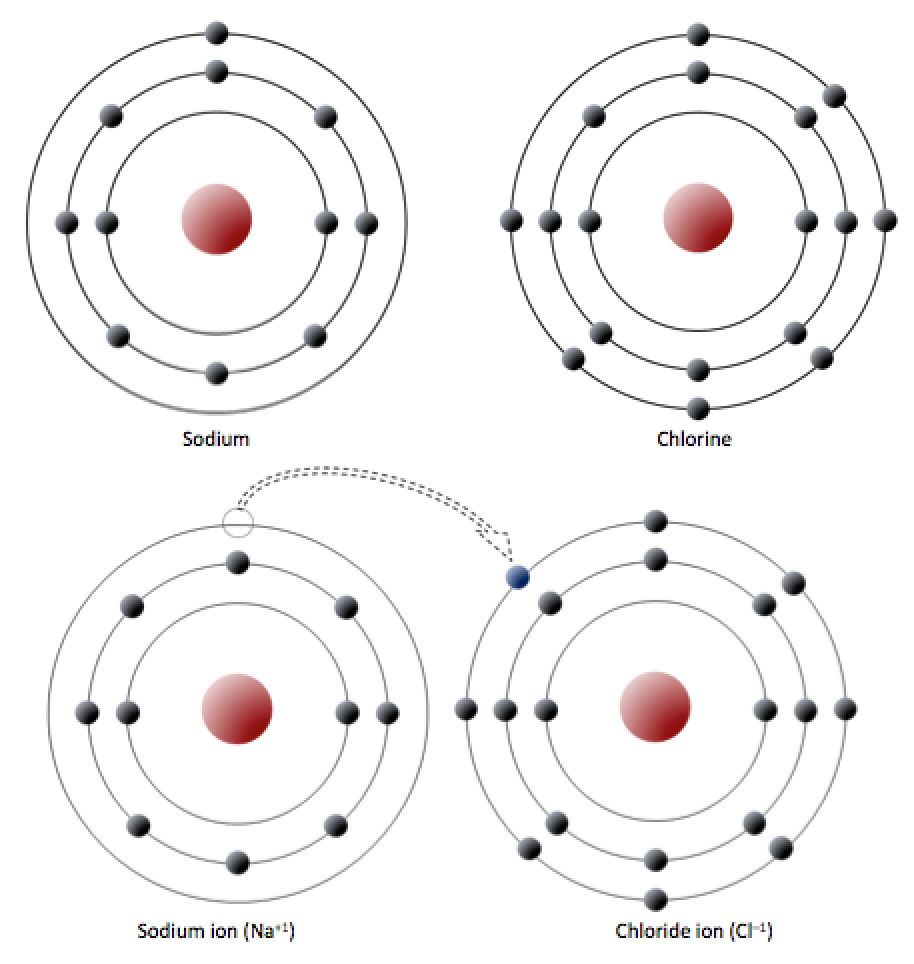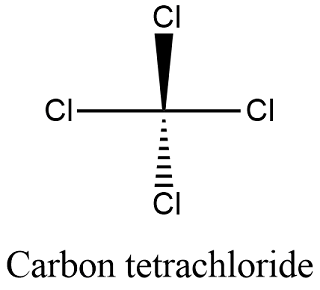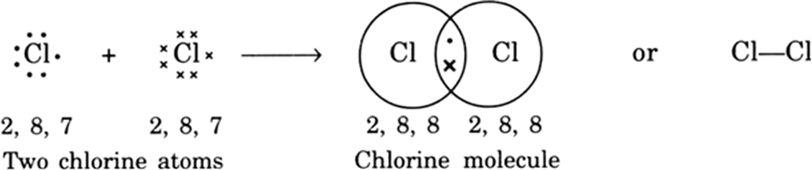
Explain the formation of covalent bonds in (i) chlorine molecule; (ii) carbon tetrachloride and (iii) ammonia. from Science Carbon and its Compounds Class 10 Jammu and Kashmir Board

Electron-Withdrawing Effects in the Photodissociation of CH2ICl To Form CH2Cl Radical, Simultaneously Viewed Through the Carbon K and Chlorine L2,3 X-ray Edges | Journal of the American Chemical Society
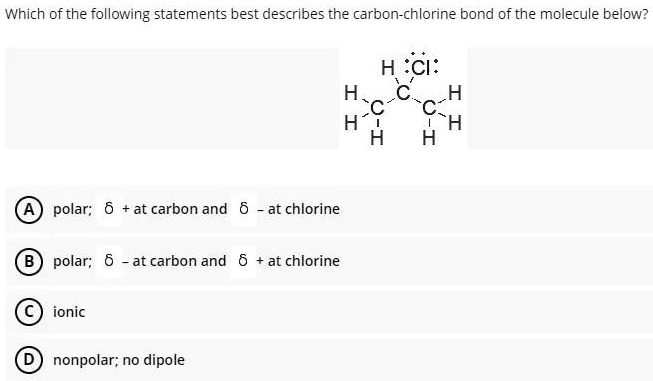
SOLVED: Which of the following statements best describes the carbon-chlorine bond of the molecule below? H :Cl: H C C- H C H H H polar; 6 +at carbon and at chlorine

Explain the formation of Covalent compound.i) Chlorine Moleculesii) Carbon Tetra Chlorideiii) Ammonia - Brainly.in

How many chlorine atoms have to combine with a carbon atom to complete its octet during the formation of carbon tetrachloride

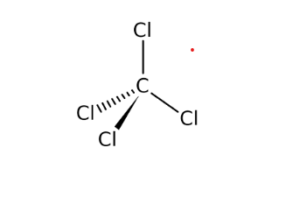
Carbon has four valence electrons, and chlorine has seven valence electrons. If carbon covalently bonds - Brainly.com

Carbon, hydrogen, oxygen, nitrogen, chlorine, and tin atoms are shown... | Download Scientific Diagram

Diagrammatic summary of the procedure for carbon and chlorine stable... | Download Scientific Diagram

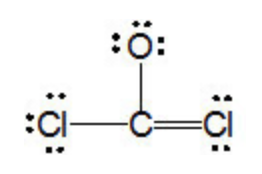
A compound composed of only carbon and chlorine is 85.5% chlorine by mass. propose a lewis structure for - Brainly.com