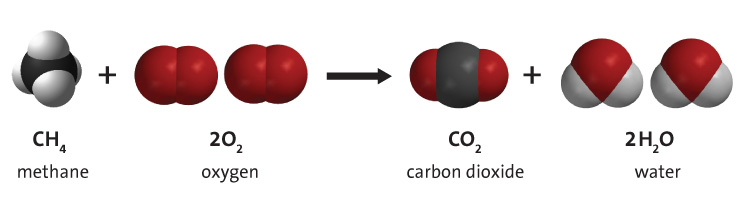
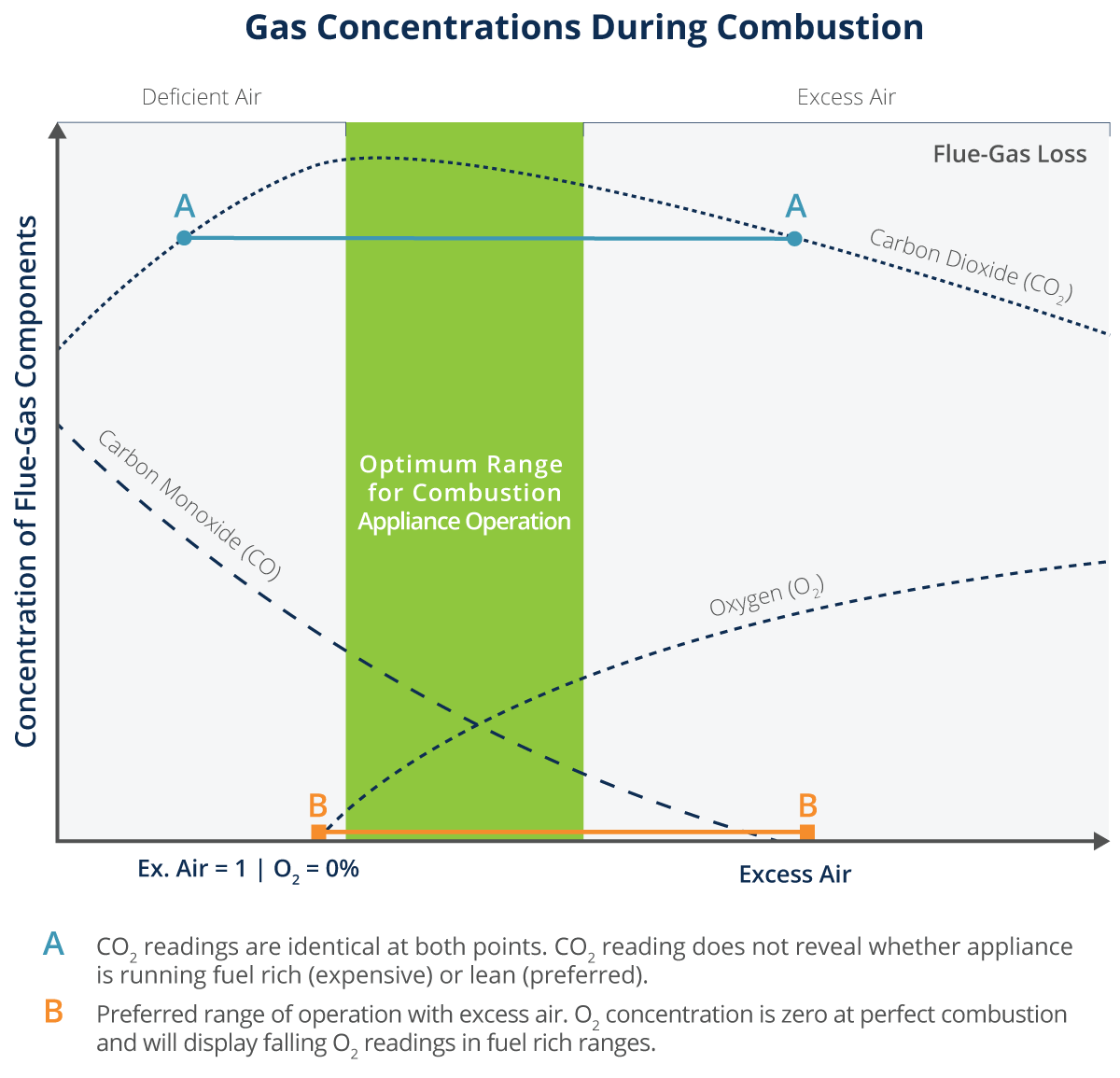
On combustion carbon forms CO and CO2 The heat of formation of CO2 is - 393.5 kJ at of CO is - 110.5 kJ .The heat combustion of CO is

Enthalpy of combustion of carbon to CO2 is -393.5KJ mol-1. Calculate the heat released upon..... - YouTube

Q12 How would you prove experimentally that a Carbon dioxide does not support combustion b Is slight...
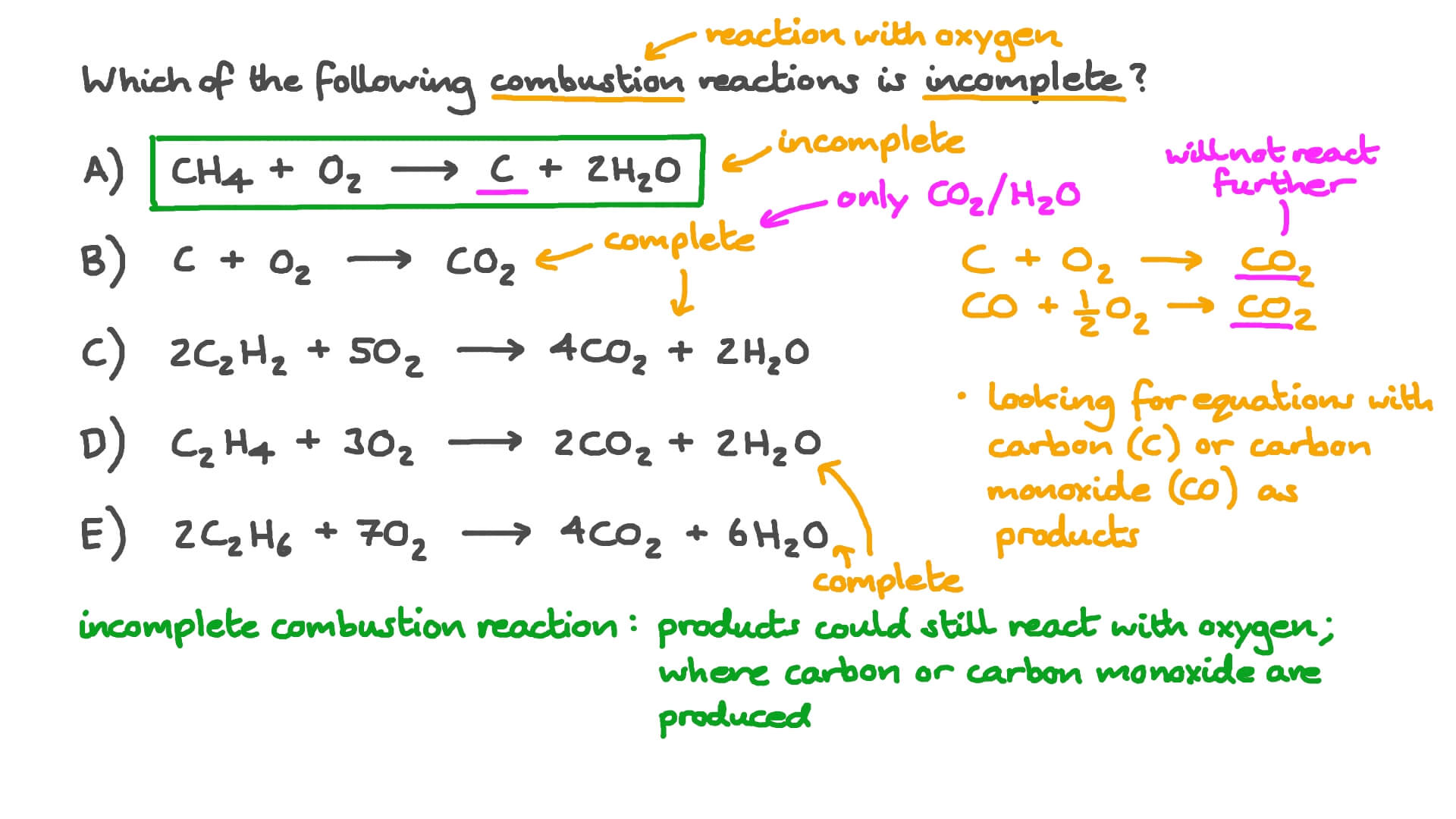
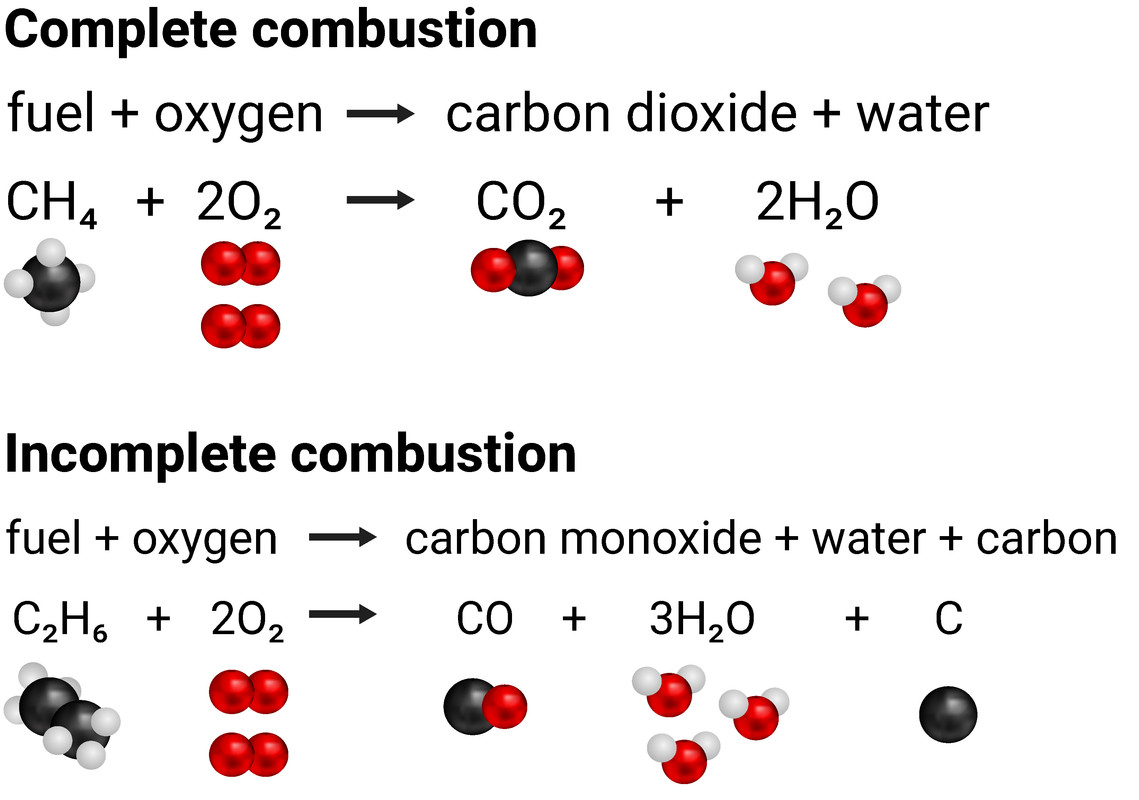
Combustion always produces carbon dioxide and water”: a discussion of university chemistry students' use of rules in place of principles - Chemistry Education Research and Practice (RSC Publishing) DOI:10.1039/C4RP00089G
the enthalpies of combustion of carbon and carbon monooxide are 393.5kJ and 283kJ ,respectively the enthalpy of formation of carbon monoxide 1. 676.5kJ 2. 110.5kJ 3.110.5kJ 4.676.5

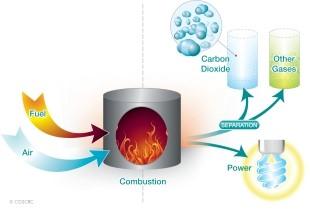
Energies | Free Full-Text | A Critical Review of CO2 Capture Technologies and Prospects for Clean Power Generation
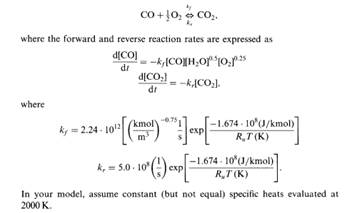
Solved) - Develop a model of the combustion of carbon monoxide with moist... - (3 Answers) | Transtutors

Enthalpy of combustion of carbon to CO2 is - 393.5 KJ/mole. The heat released upon the formation of 35.2g of CO2 from carbon and dioxygen gas is.
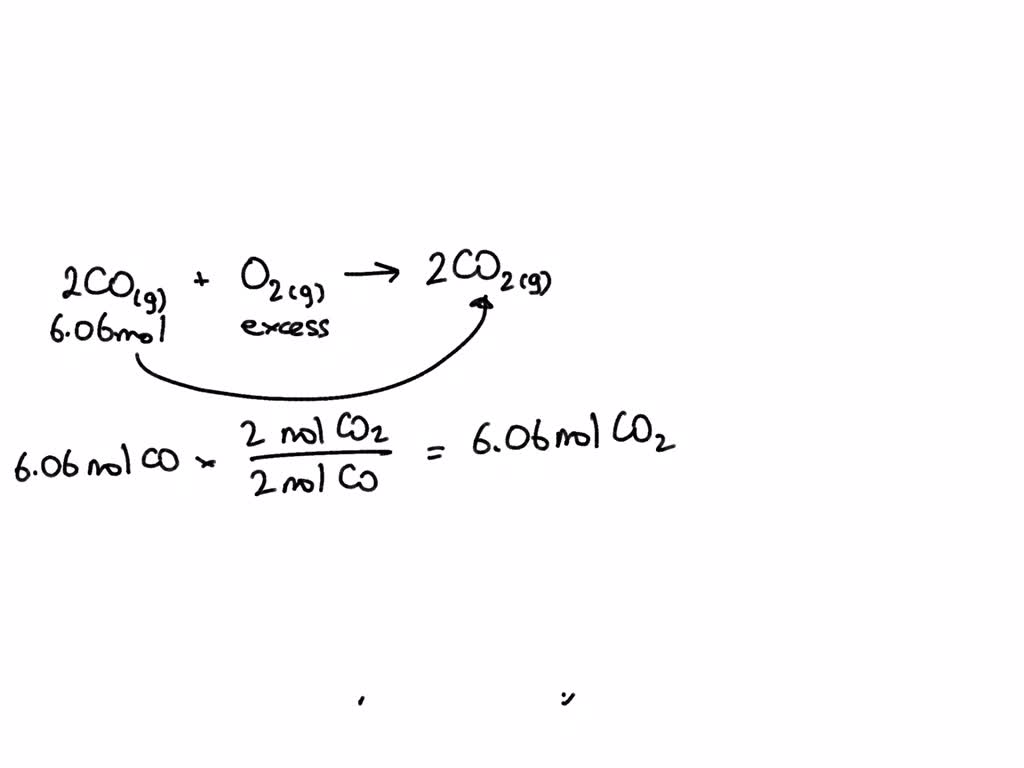
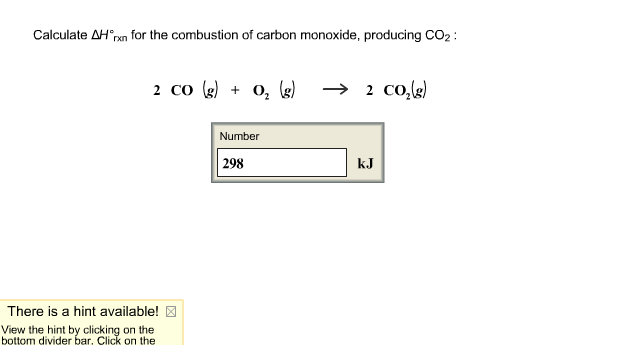
SOLVED: Consider the combustion of carbon monoxide (CO) in oxygen gas: 2CO(g) + O2(g) → 2CO2(g) Calculate the number of moles of CO2 produced if 6.06 moles of CO are reacted with
62.The Enthalpies of combustion of carbon and carbon monoxide are 390 kJ and 278kJ respectively. The enthalpy of formation of carbon monooxide is? a) 669 kJ b) 112 kJ c) 112 kJ d) 668 kJ