Carbon Disulfide Burning in Liquid Oxygen | Karbon disulfidin maye oksigendə yanması CS2 + 3 O2 → CO2 + 2 SO2 © ChemicalForce | By Azerbaijan Chemical Society | Facebook
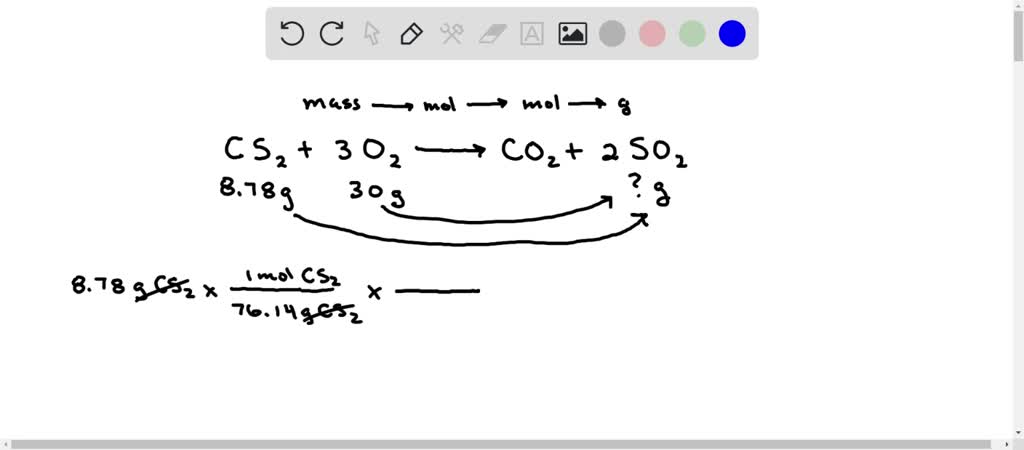
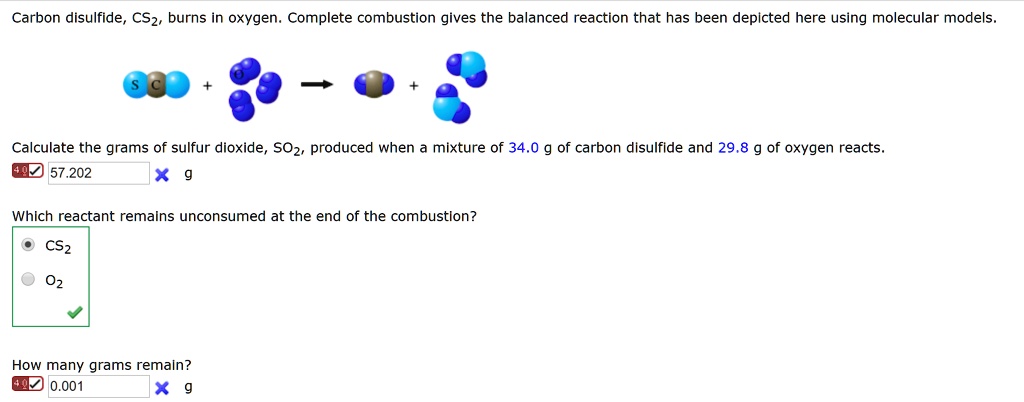
SOLVED: The combustion of carbon disulfide in the presence of excess oxygen yields carbon dioxide and sulfur dioxide according to the following UNBALANCED reaction: CS2 (g) + 3O2 (g) → CO2 (g) +

The enthalpy of combustion of carbon and carbon monoxide are - 393.5 and - 283 kJ/mol respectively. The enthalpy of formation of carbon monoxide per mole is:

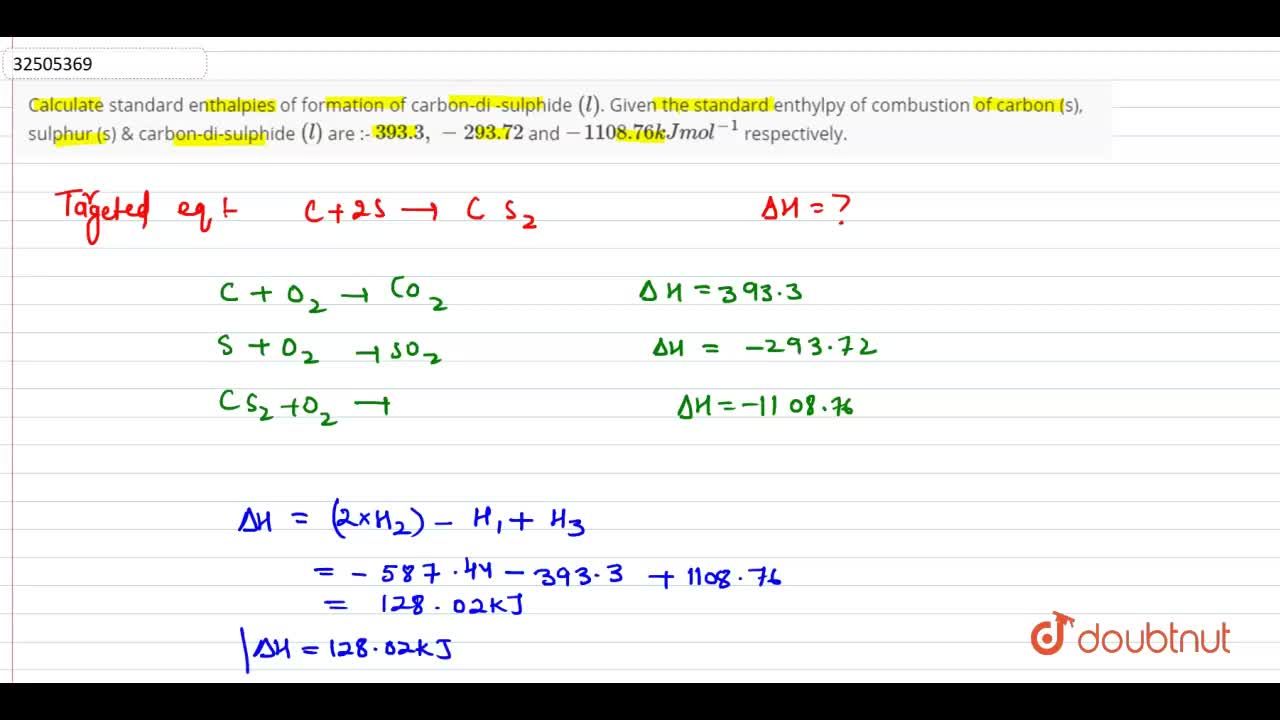
The standard enthalpy of formation of carbon - di - sulphide (l) is:Given the standard enthalpy ofcombustion of carbon (s) , sulphur (s) & carbon - di - sulphide (l) are : - 393.3, - 293.72 and - 1108.76 kJ mol^-1 respectively.

SOLVED: Enter your answer in the provided box: Calculate the standard enthalpy of formation of carbon disulfide (CS2) from it's elements, given that C(graphite) O,lg) COzkg) AH 393.5 kJlmol S(rhombic) O-(g) SOz(g)
![PDF] Oxidation chemistry of carbon disulfide (CS2) and its interaction with hydrocarbons in combustion processes | Semantic Scholar PDF] Oxidation chemistry of carbon disulfide (CS2) and its interaction with hydrocarbons in combustion processes | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a6bc9690bc4f626d516ce0966c1f087914aafa2d/29-Table2.1-1.png)
PDF] Oxidation chemistry of carbon disulfide (CS2) and its interaction with hydrocarbons in combustion processes | Semantic Scholar
![PDF] Oxidation chemistry of carbon disulfide (CS2) and its interaction with hydrocarbons in combustion processes | Semantic Scholar PDF] Oxidation chemistry of carbon disulfide (CS2) and its interaction with hydrocarbons in combustion processes | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a6bc9690bc4f626d516ce0966c1f087914aafa2d/50-Figure2.10-1.png)
PDF] Oxidation chemistry of carbon disulfide (CS2) and its interaction with hydrocarbons in combustion processes | Semantic Scholar
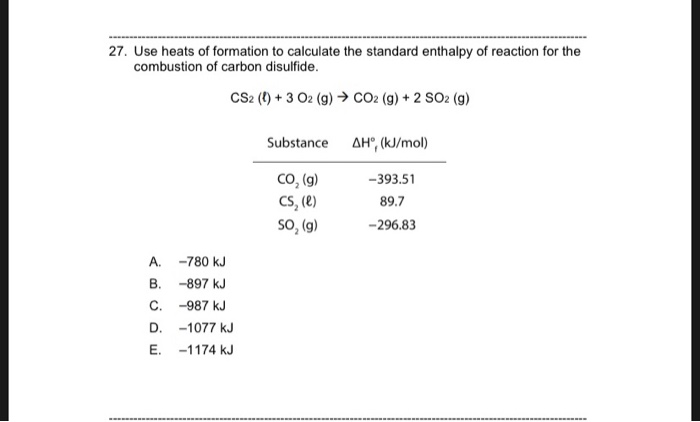
43. Calculate standard heat of formation of CS2. Given that standard heat of combustion of C, S and CS2 are –393.3, –293.72 and –1108.76 kJ mol–1

Calculate the standard heat of formation of carbon disulphide (l). Given that the standard heats of - YouTube
![PDF] Oxidation chemistry of carbon disulfide (CS2) and its interaction with hydrocarbons in combustion processes | Semantic Scholar PDF] Oxidation chemistry of carbon disulfide (CS2) and its interaction with hydrocarbons in combustion processes | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a6bc9690bc4f626d516ce0966c1f087914aafa2d/55-Figure2.13-1.png)
PDF] Oxidation chemistry of carbon disulfide (CS2) and its interaction with hydrocarbons in combustion processes | Semantic Scholar

The heat of combustion of carbon to CO2 is - 393.5 kJ/mol. The heat released upon formation of 35.2 g of CO2 from carbon and oxygen gas is:

OneClass: the thermochemical equation for the combustion of carbon disulfide is: CS2(l)+3O2(g)->CO...
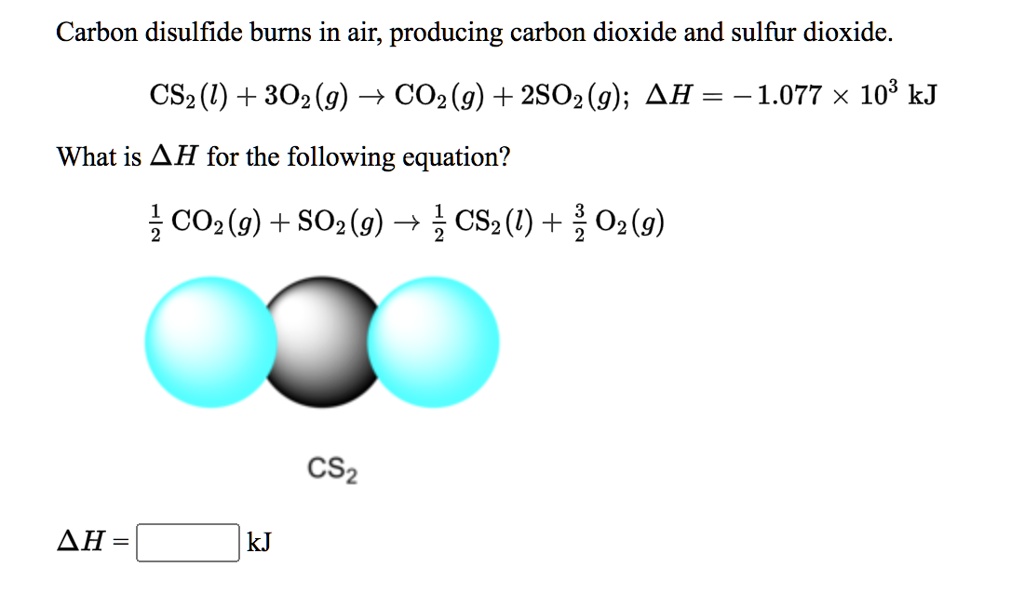
SOLVED: Carbon disulfide burns in air; producing carbon dioxide and sulfur dioxide. CS2 (U) + 302(g) + CO2 (g) + 2802 (9); 4H = -1.077 X 103 kJ What is AH for