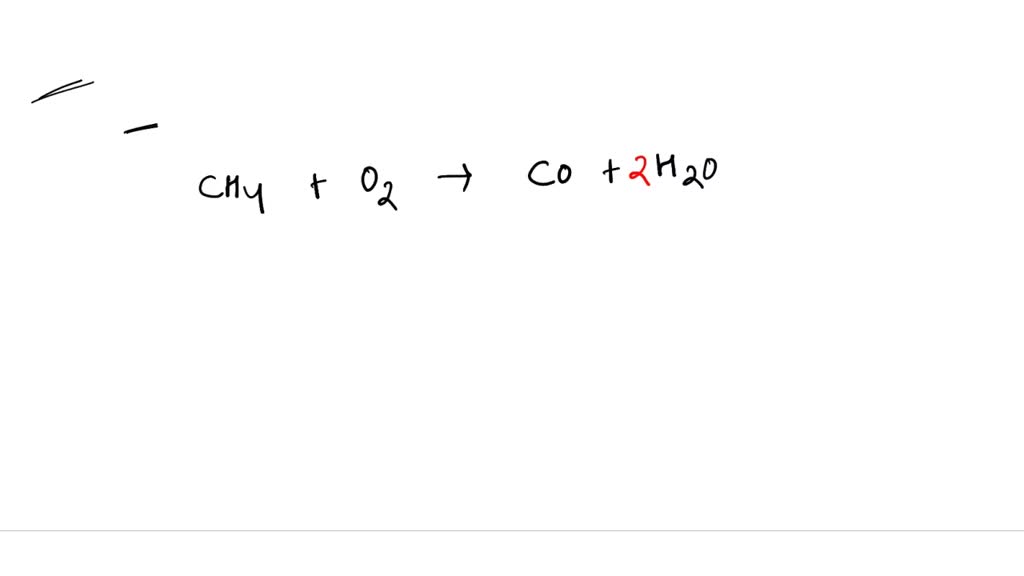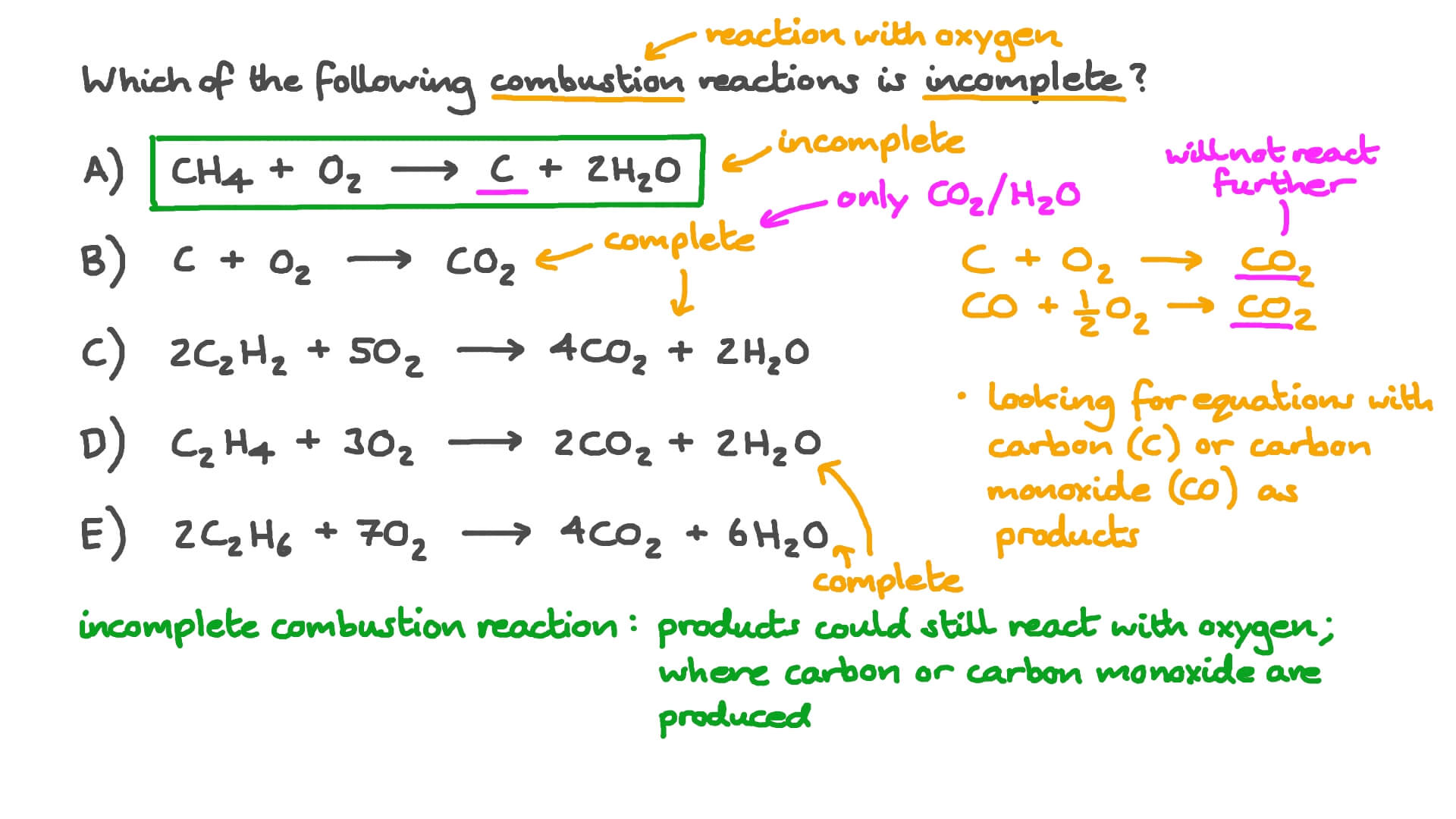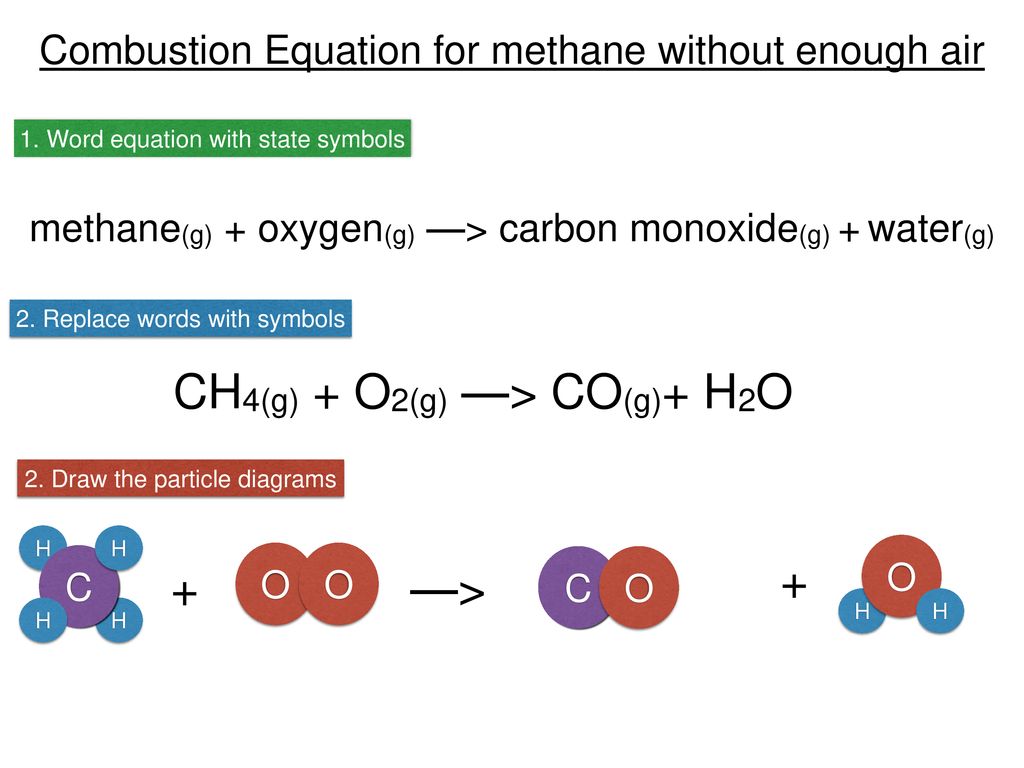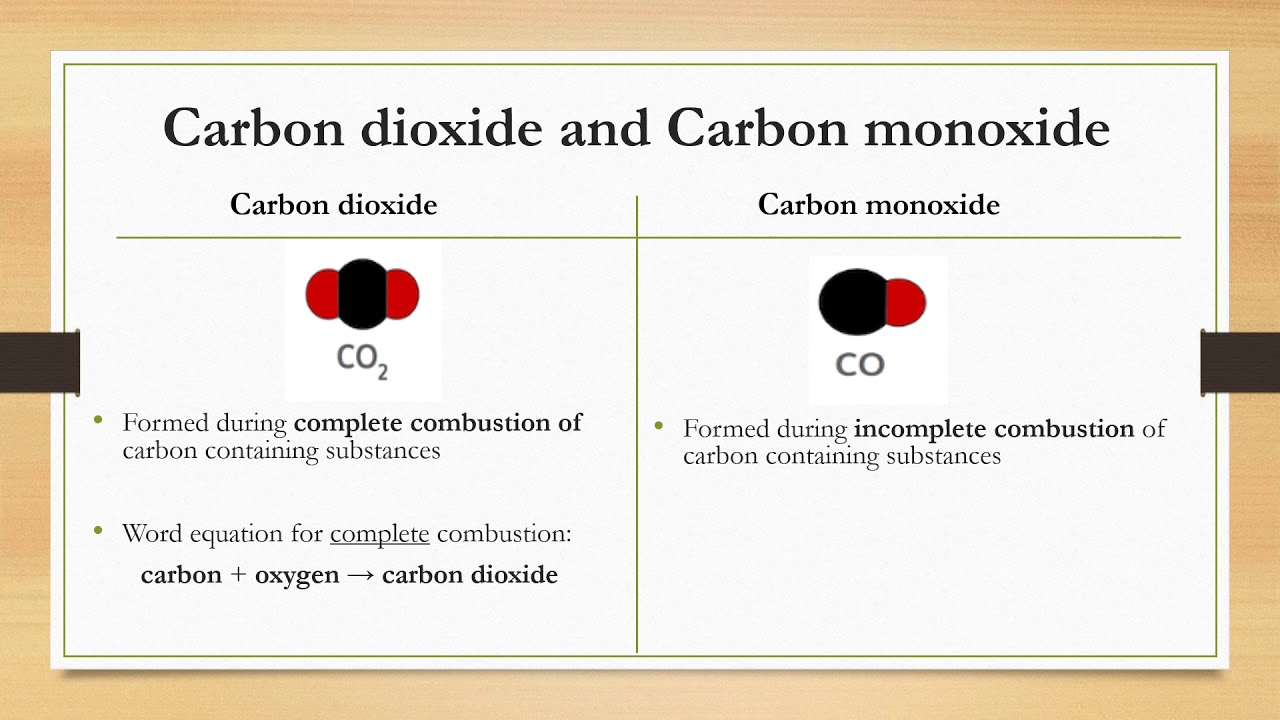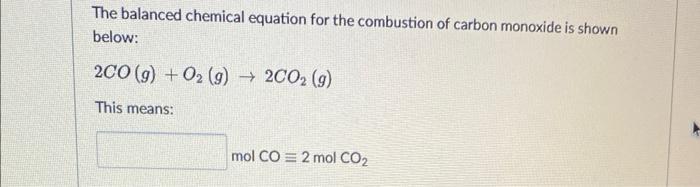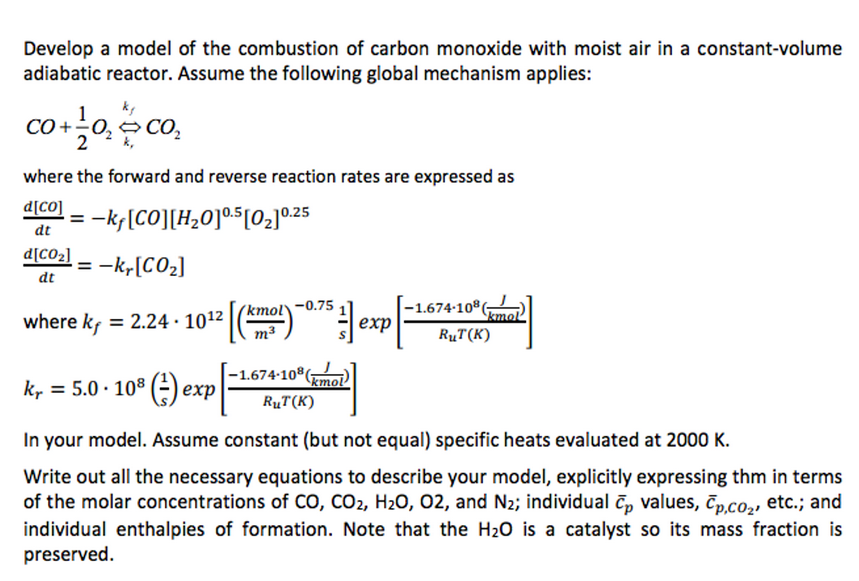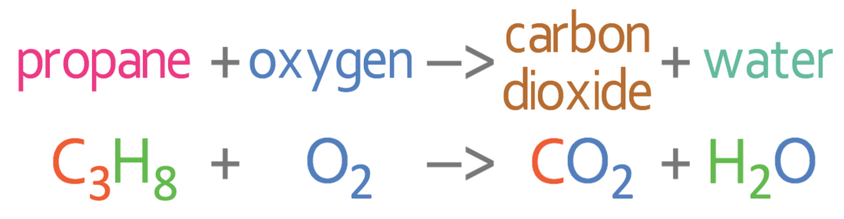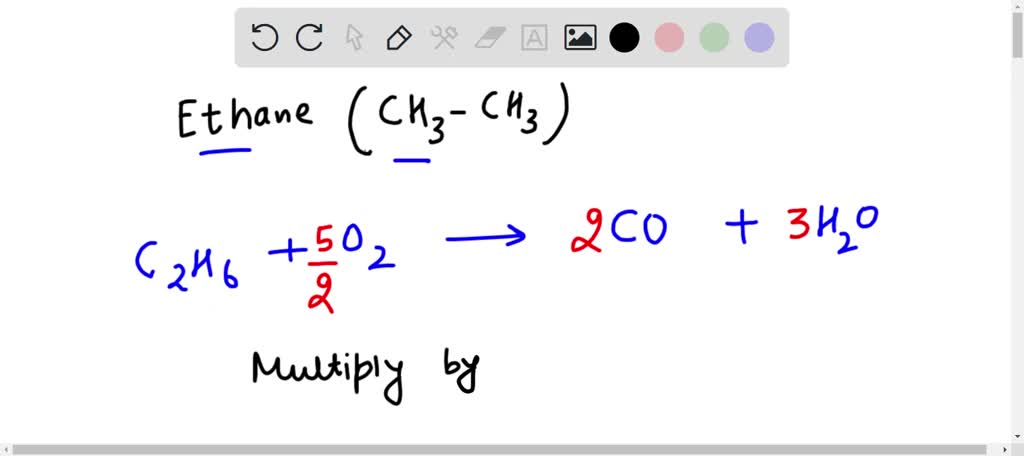
SOLVED: Write a balanced equation for the incomplete combustion of ethane (CH3CH3) to form carbon monoxide as one product.

SOLVED: In oxygen-deficient environments, the carbon in coal reacts with oxygen to form carbon monoxide, COCO, instead of carbon dioxide, CO2CO2. Write the balanced chemical equation for the incomplete combustion of carbon

The heat of the combustion of graphite and carbon monoxide respectively are 393.5 kJ mol1 and 283 kJ mol ^-1 . Thus heat of formation of carbon monoxide in kJ mol ^-1 is:

The enthalpies of formation of CO and `CO_(2)` are `-110.5 KJ mol^(-1)` and `-393.5 KJ mol^(-1)` - YouTube
The enthalpies of combustion of carbon and carbon monoxide are -393.5 and –283kJ mol^–1 respectively. - Sarthaks eConnect | Largest Online Education Community

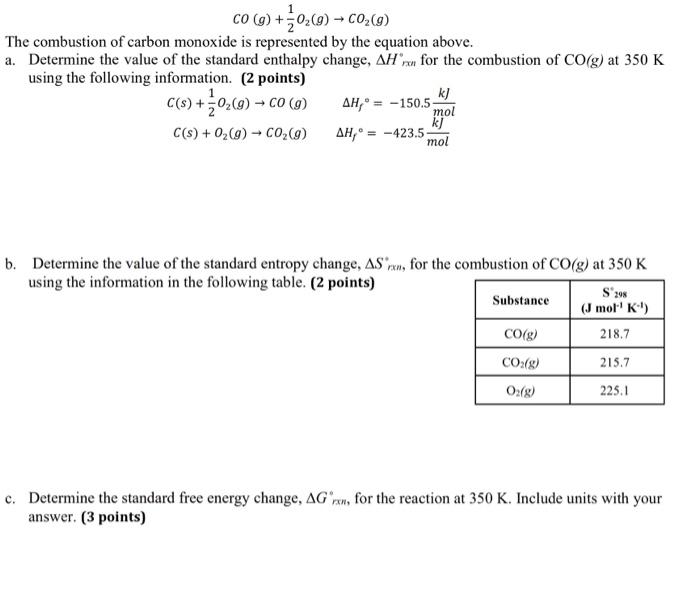
OneClass: The combustion of carbon monoxide is represented by the equation above Determine the value ...

The enthalpy of combustion of carbon and carbon monoxide are - 393.5 and - 283 kJ/mol respectively. The enthalpy of formation of carbon monoxide per mole is:
the enthalpies of combustion of carbon and carbon monooxide are 393.5kJ and 283kJ ,respectively the enthalpy of formation of carbon monoxide 1. 676.5kJ 2. 110.5kJ 3.110.5kJ 4.676.5
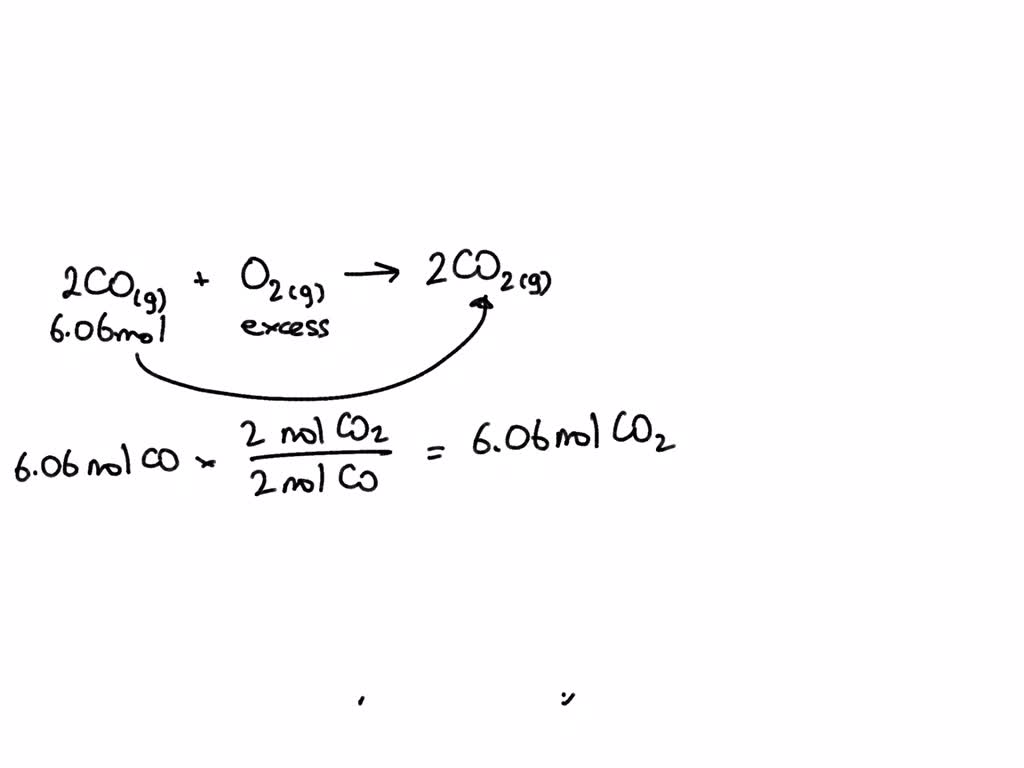
SOLVED: Consider the combustion of carbon monoxide (CO) in oxygen gas: 2CO(g) + O2(g) → 2CO2(g) Calculate the number of moles of CO2 produced if 6.06 moles of CO are reacted with