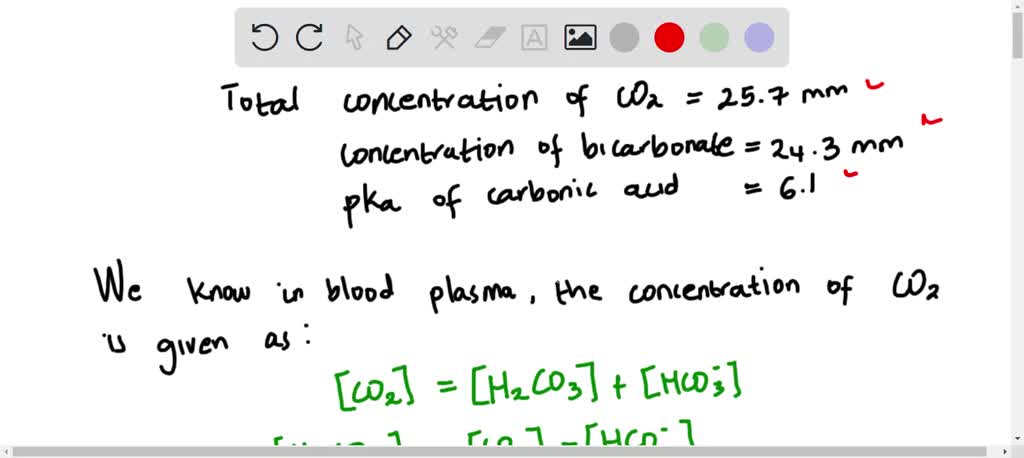
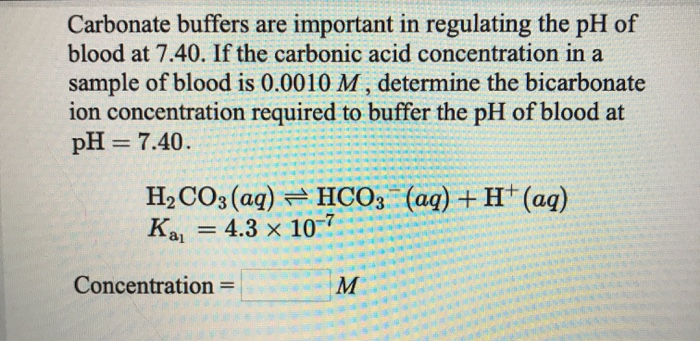
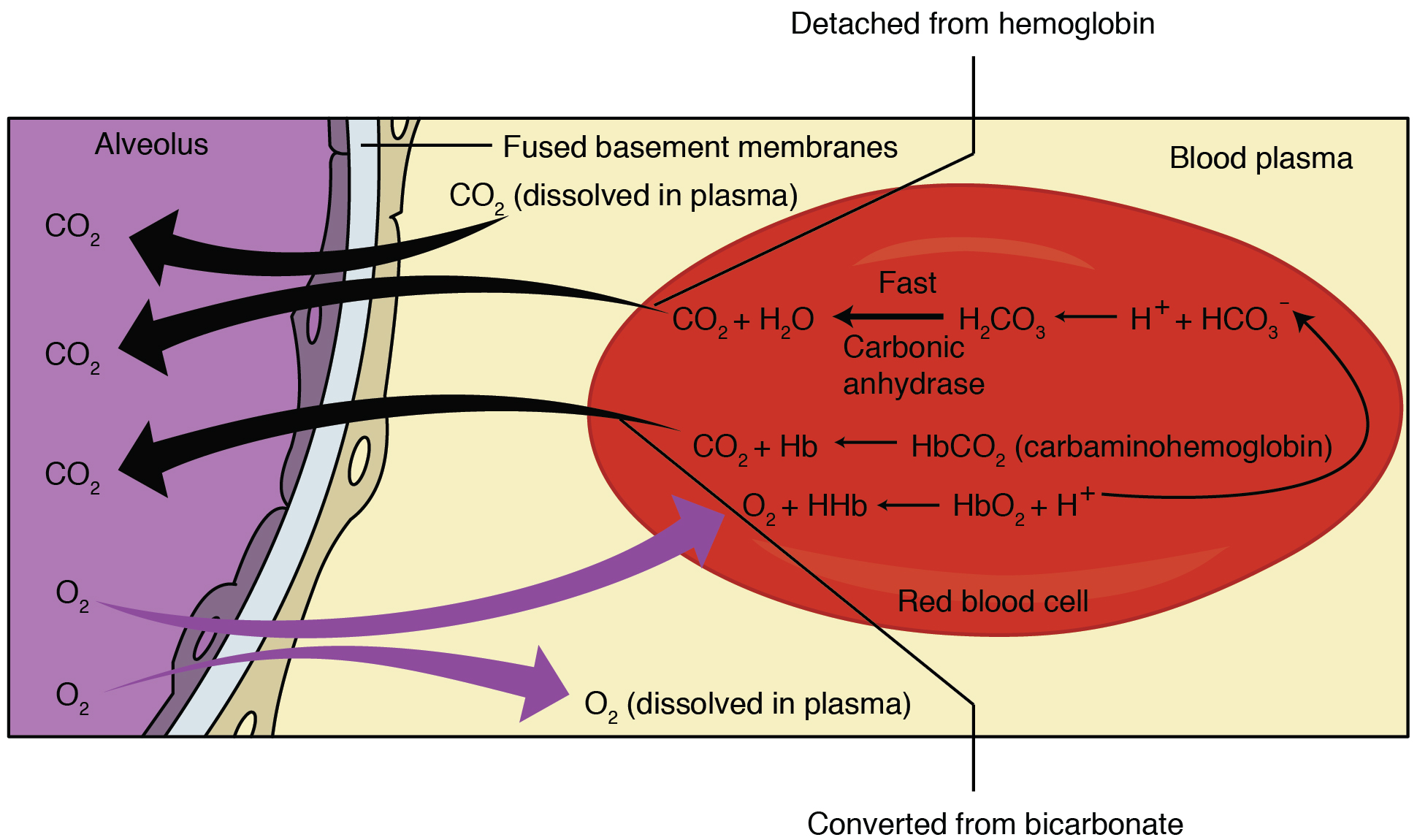
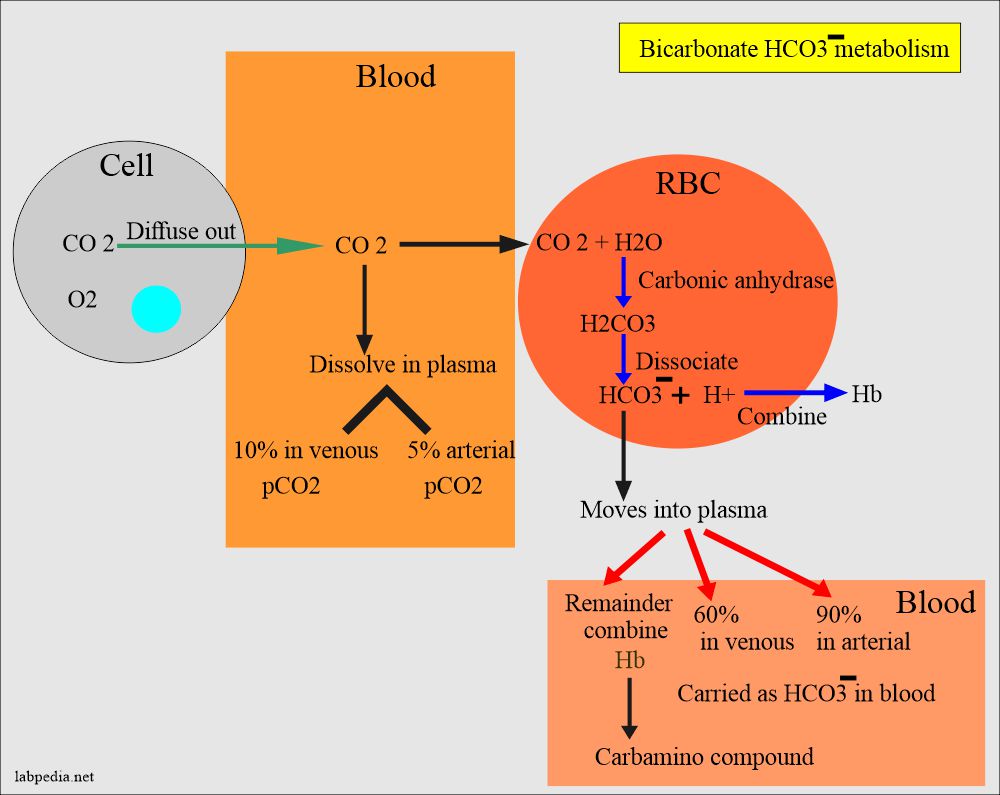
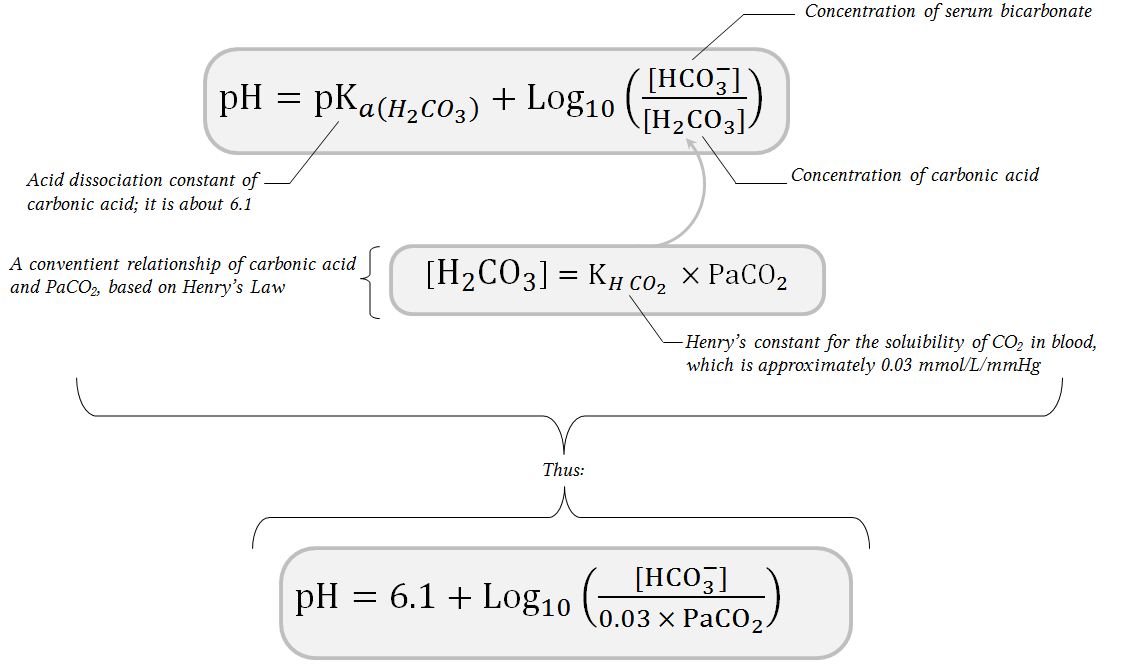
SOLVED: The pH of a bicarbonate-carbonic acid buffer is 6.62. Calculate the ratio of the concentration of carbonic acid ( H2CO3 ) to that of the bicarbonate ion ( HCO3− ). (

Percentage of free carbonic acid from total carbonic acid Q c depending... | Download Scientific Diagram
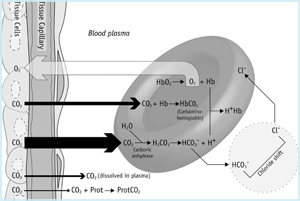
Why is human blood basic (pH 7.4) if it contains a buffer of carbonic acid (H₂CO₃) and bicarbonate anion (HCO₃⁻) in order to maintain blood pH between 7.35 and 7.45? - Quora

The pH of the blood is maintained by the carbonic acid - bicarbonate buffer. The pH of the buffer is :

SOLVED: One of the buffers that contribute to pH stability in human blood is carbonic acid (H 2C0 3). Carbonic acid is a weak acid that; when placed in a aqueous solution;