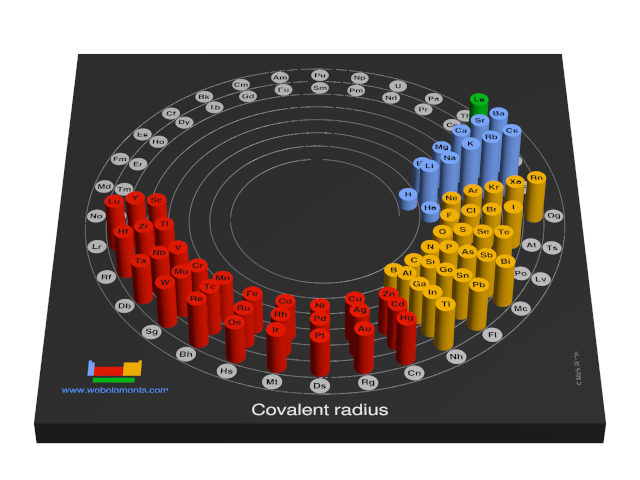
![a. Covalent radii [4] of the atoms: C, N, H, O & P which account [3]... | Download Scientific Diagram a. Covalent radii [4] of the atoms: C, N, H, O & P which account [3]... | Download Scientific Diagram](https://www.researchgate.net/profile/Raji-Heyrovska/publication/1908623/figure/fig1/AS:279452455063564@1443638065279/a-Covalent-radii-4-of-the-atoms-C-N-H-O-P-which-account-3-for-all-the-bond_Q320.jpg)
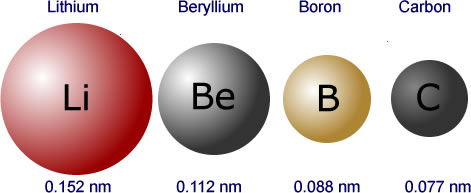
a. Covalent radii [4] of the atoms: C, N, H, O & P which account [3]... | Download Scientific Diagram
Assuming covalent radii to be additive property; calculate the iodine-io-dine distances in - Sarthaks eConnect | Largest Online Education Community

The value of covalent bonf length of Si - C is 1.93 A . Covalent radius of carbon atom is 0.77 A . Calculate the covalent radius of silicon atom.

Planar Hexacoordinate Carbons: Half Covalent, Half Ionic - Leyva‐Parra - 2021 - Angewandte Chemie International Edition - Wiley Online Library
What is the derivation for the covalent radius formula for hetero atomic covalant compounds? - Quora